AIIMS Previous Paper - 2013 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Previous Paper - 2013
To obtain a p-type germanium semiconductor, it must be doped with
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The magnifying power of a compound microscope is high If
To double the covering range of a TV transmitter tower, its height should be made
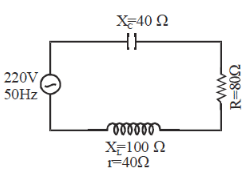
An alternatmg voltage V = V0 sin ωτ is applied across a circuit. As result I = I0sin(ωτ)- π/2) flows ill it. The power consumed per cycle is
A slab consist of two portions of different materials of same thickness and having the conductivities K1 and K2. The equivalent thermal conductivity of the slab is
A prism is made up 0f material of refractive index √3. The angle of prism is A. If the angle of minimum deviation is equal to the angle of the prism, then the value of A is
The half-life of a radioactive substance is 10 day. This means that
A source and an observer are moving towards each other with a speed equal to υ/2, where υ is the speed of sound. Thesource is emitting sound of frequency n.The frequency heard by one observer will be
Velocity of sound waves in air is 330 m/s. For a particular sound in air a path difference of 40 cm is equivalent to phase difference of 1.6π. The frequency of the wave is
A particle is executing linear simple harmonic motion amplitude A. What fraction of the total energy is kinetic when the displacement is half the amplitude.
Two simple harmonic motions are represented by y1 = 4sin(πt– π/2) and y2 = 3cos(4πt). The resultant amplitude is
Which one of the following bonds produces a solid that reflects light in the visible region and whose electrical conductivity decreases with temperature and hs high melting point?
When 20 J of work was done on gas. 40 J of heat energy was released If the initial energy of the gas was 70 J what is the final internal energy’?
The value of g at a particular point is 9.8 ms-2 Suppose the earth suddenly shrinks uniformly to half it resent size without losing any mass . The value of g at the same point (distance of the point from the centre of earth does not change) Will now be
A spherical ball is dropped in a long column of viscous liquid. Which of the following graphs represent the variation of (i) gravitational force with time (ii) viscous force with time (iii) net force acting on the ball with time?
The Young’s modulus of a wire of length L the radius r is y newton per square meter lf the length is reduced to L/2 and radius L/2 Its Young’s modulus will be
A boy of mass m stands on one end of a wooden plank of length L. and muss M The plank is floating on water, If the boy walks from one end of the plank to the other end at a constant speed theresulting displacement of the plank is given by
A sphere of solid material of relative density 9 has a concentric spherical cavity and Just sinks in water. If the radius of sphere be R. then the radius of cavity (r) will be related to R as
Average value of kinetic energy and potential energy over entire time period in a SHM is
In which of the state- shown in figure, is potential energy of a electric dipole maximum?
A car is travelling with a linear velocity u on a Circular road of radius r. If It is increasing its speed at the rate of a m/s2 then the resultant acceleration will be
A uniform magnetic field parallel to the plane of paper, exsisted in space initially directed from left to right. When a bar of soft Iron is placed in the field parallel to it, the lines of force passing through It will be represented by figure.
A body starts from rest and moves with a uniform acceleration. The ratio of the distance covered in the n th second to the distance covered in nsecond
Wind blowing from South at 10 m/s but to a cyclist it appears to be blowing from the East at 10 m/s The cyclist has a velocity
The ratio of radius of two bubbles is 2: 1. What is the ratio excess pressure inside them?
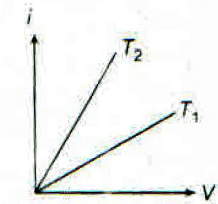
The current. i and voltage V graphs for a given metallic wire at two different temperatures T1 and T2 are shown In the figure. It is concluded that
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|