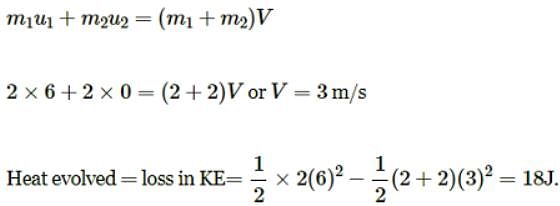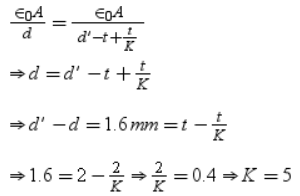SRMJEE Mock Test (Med) - 1 - JEE MCQ
30 Questions MCQ Test SRMJEEE Subject Wise & Full Length Mock Tests 2024 - SRMJEE Mock Test (Med) - 1
A low-loss transformer has 230 volts applied to the primary and gives 4.6 volts in the secondary. Secondary is connected to a load which draws 5 ampere of current. The current (in ampere) in the primary is
The particles emitted by radioactive decay are deflected by magnetic field. The particles will be
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A car is initially at rest on a circular track that has a radius of 40 m. The car then starts to move counter clockwise around the track, accelerating at constant angular acceleration of 1.0 rad/s2. The time it takes for the car to move half of the way around the track is
If the ratio of the concentration of electrons to that of holes in a semiconductor is 7/5 and the ratio of the currents is 7/4, then what is the ratio of their drift velocities?
A body of mass 2Kg moving with a velocity of 6 ms-1 strikes inelastically another body of same mass at rest. The amount of the heat evolved during collision is
According to Hooke's law of elasticity, the ratio of stress to strain
Reactance of a capacitor of capacitance C μ F for a.c. of frequency 400/π Hz is 25 Ω. The value of C is
The plates of a parallel plate capacitor are charged upto 200 volts. A dielectric slab of thickness 4 mm is inserted between its plates. Then to maintain the same potential difference between the plates capacitor, the distance between the plates is increased by 3.2 mm. The dielectric constant of dielectric slab is
A block of 1 kg is stopped aganist a wall by aplying a force F perpendicular to the wall. If μ = 0.2 then minimum value of F will be
Choose the correct statement from the following. The radius of the orbit of a geostationary satellite depends upon
Consider two rods of same length and different specific heatrs (s₁ and s₂) conductivities k₁ and k₂ and areas of cross section (A₁ and A₂) and both giving temperature T₁ and T₂ at their ends. If the rate of heat loss due to conduction is equal, then
At 27oC temperature, the kinetic energy of an ideal gas is E1. If the temperature is icnreased to 327oC, then kinetic energy would be
Consider the following statements concerning electrons :(I) Electrons are universal constitutents of matter (II) J.J. Thomson received the very first Nobel Prize in Physics for discovering the electron (III) The mass of the electron is about 1/2000 of that of a neutron (IV) According to Bohr the linear momentum of the electron is quantised in the hydrogen atom. Which of the above statements are NOT correct ?
A body of mass 60 kg is dragged with just enough force to start moving on a rough surface with coefficients of static and kinetic frictions 0.5 and 0.4 respectively. On applying the same force, what is the acceleration :
Magnetic field to a current carrying conductor is propotional to
Oil spreads over the surface of water, whereas water does not spread over the surface of oil, because
A passenger train is moving at 5 m−s-1. An express train is travelling at 30 m−s-1, on the same track and rear side of the passenger train at some distance. The driver in express train applied brakes to avoid collision. If the retardation due to brakes is 4 m−s-1, the time in which the accident is avoided after the application of brakes is
A 100 kg car is moving with a maximum velocity of 9 m/s across a circular track of radius 30 m. The maximum force of friction between the road and the car is
A car of mass 400 kg travelling at 72 kmph crashes into a truck of mass 4000kg and travelling at 9 kmph in same direction. The car bounces back at a speed of 18 kmph. The speed of the truck after the impact is
A coin is placed on a horizontal platform, which undergoes vertical simple harmonic motion of angular frequency ω. The amplitude of oscillations is gradually increased. The coin will have contact with the platform for the first time. (i) at the higher position of the platform (ii) at the mean position of the patform (iii) for an amplitude of g/ω2 (iv) for an amplitude of √2/ω
A stone thrown at an angle θ to the horizontal reaches a maximum height h. The time of flight of the stone is
A ray of light strikes a material's slab at an angle of incidence 600 . If total reflected and refracted rays are perpendicular to each other, the refractive index of the materials is
The moment of inertia of a sphere of radius R and mass M about a tangent to the sphere is
Consider the following statements about dimensions
P:Force=acceleration due to gravity x mass
Q:Electric charge=current x time
R:Magnetic flux=Electric voltage x time
The correct statements are :
Force necessary to pull a circular plate of 5 cm radius from water surface for which surface tension is 75 dynes/cm,is
The negative Zn pole a Daniell cell, sending a constant current through a circuit, decreases in mass by 0.13g in 30 minutes. If the electrochemical equivalent of Zn and Cu are 32.5 and 31.5 respectively, the increase in the mass of the positive Cu pole in this time is
The pressure of a given mass of gas is 100 cm at 127oC . The pressure of the same mass of gas having the same volume at 27oC is ......
|
1 videos|1 docs|68 tests
|
|
1 videos|1 docs|68 tests
|