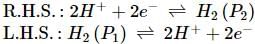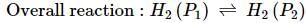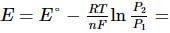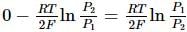JIPMER Chemistry Mock Test - 3 - NEET MCQ
30 Questions MCQ Test JIPMER: Subject wise Tests & Practice Mock Tests 2025 - JIPMER Chemistry Mock Test - 3
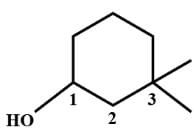
The structural formula of 2-methyl 2-butene is .......
Ethyl bromide on treatment with alocoholic KOH gives
The first emission line in the atomic spectrum of hydrogen in the Balmer series appears at
The atomic number of an element is 35. What is the total number of electrons present in all the p-orbitals of the ground state atom of the element
Which one of the following is not an isoelectronic pair
A one litre flask is full of brown bromine fumes. The intensity of brown colour of vapour will not decrease appreciably on adding to the flask some
For a given reaction t1/2 = (1/ka). The order of reaction is
The compound with carbon uses only its sp3 hybrid orbitals for bond formation is
The bond dissociation energies of H₂,Cl₂ and HCl are 104, 58 and 103 kcal respectively. The enthalpy of formation of HCl gas will be
Which pair of atomic numbers represent elements which are both s-block elements
Which of the following is an example of a double salt?
What will be the e.m.f. of the given cell?
Pt | H2(P1)| H+(aq)|H2(P2)|Pt
At which one of the following temperature-pressure conditions, the deviation of a gas from ideal behaviour is expected to be minimum ?
300 ml of a gas at 27oC is cooled to -3oC at consatnt pressure, the final volume is
The strongest reducing agent of the alkali metal is
|
4 videos|8 docs|51 tests
|
|
4 videos|8 docs|51 tests
|



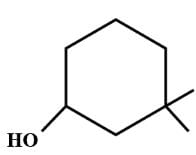 is
is