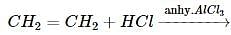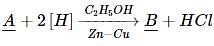JIPMER Chemistry Mock Test - 4 - NEET MCQ
30 Questions MCQ Test JIPMER: Subject wise Tests & Practice Mock Tests 2024 - JIPMER Chemistry Mock Test - 4
Replacement of Cl of chlorobenzene to give phenol requires drastic conditions but chlorine of 2, 4-Dinitrochlorobenzene is readily replaced because
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In its reaction with silver nitrate acetylene shows
When a mixture of solid NaCl, solid K₂Cr₂O₇ is heated with conc.H₂SO₄, orange red vapour are obtained. These are of the compound
An ion has 18 electrons in the outermost shell. The ion is
Which of the following sets of quantum numbers is not possible?
The value of the energy for the first excited state of hydrogen will be
Since enthalpy of elements in their natural state is taken to the zero .The value of ∆Hf of compounds
The first order rate constant for the decomposition of N₂O₅ is 6.25 x 10-4 second-1. The half-life for this decomposition is
The equilibrium constant for a reaction is 73 at 298 K. The value of standard free energy change is
Tranquilisers are substances used for the treatment of
Which of the following statement about fluorine is not correct?
How many EDTA (ethylemediaminetetra acetic acid) molecules are required to make an octahedral complex with Ca2+ ion?
Which of the following statements is false for alkali metals?
3.2 oxygen is diffused in 10 minutes. In similar conditions, 2.8 g nitrogen will diffuse in
Al is more rective than Fe but Al is less easily corroded than Fe because
The chloride of an element A gives a neutral solution in water. In the periodic table, the element A belongs to
Which of the following is the correct statement for PH₃?
|
4 videos|8 docs|51 tests
|
|
4 videos|8 docs|51 tests
|