JIPMER Chemistry Mock Test - 8 - NEET MCQ
30 Questions MCQ Test JIPMER: Subject wise Tests & Practice Mock Tests 2025 - JIPMER Chemistry Mock Test - 8
In the following reaction X and Y are respectively,
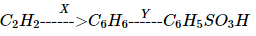
The reaction of 4-bromobenzyl chloride with NaCN in ethnol leads to
In its reaction with silver nitrate acetylene shows
An aqueous solution of colourless metal sulphate M, gives white ppt. with NH₄OH. This was soluble in excess of NH₄OH. On passing H₂S through this solution a white ppt. is formed. The metal M in the salt is
If the radius of first Bohr orbit be a₀, then the radius of the third orbit of hydrogen would be
The spectrum of He is expected to be similar to that of
Which of the following does not have sp2-hybridised carbon?
The bond order of individual C - C bond in benzene is
According to the Fajan's rule, covalent bond is favoured by
The favourable conditions for a spontaneous reaction are
Consider the reaction N2(g) + 3H2(g) → 2NH3(g). The equality relationship between (d[NH3]/dt) and -(d[H2]/dt) is
The minimum energy required by molecules to react is called
When 10gram of methane is completely burnt in oxygen, the heat evolved is 560kJ. What is the heat of combustion (in kJ/mole) of methane?
If a dye absorbs green colour in the visible region, then it will appear
Which of the following compounds will exhibits geometrical isomerism ?
Which of the following ions are paramagnetic in character?
Among the following complexes, which has a magnetic moment of 5.9 BM?
At STP,1.12 litre of H₂ is obtained on flowing a current for 965 seconds in a solution.The value of current is
Refractory metals are used in construction of furnaces because
Which of the following mixture of gases does not obey Dalton's law of partial pressure ?
Which one of the following indicates the value of the gas constant R ?
The number of sigma and pi ( π ) bonds present in 'inorganic benzene' respectively are
Oxalic acid reacts with conc. H2SO4 to give a mixture of two gases. When this mixture is passed through caustic potash. One of the gases is absorbed. What is the product formed by the absorbed gas with caustic potash
Two oxides of nitrogen, NO and NO2 react together at 253K an form a compound of nitrogen X. X reacts with water to yield another compound of nitrogen Y. The shape of the anion of Y molecule is
|
4 videos|8 docs|51 tests
|
|
4 videos|8 docs|51 tests
|


















