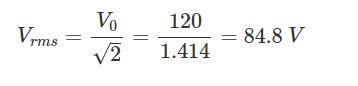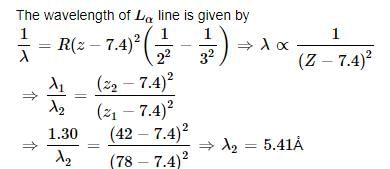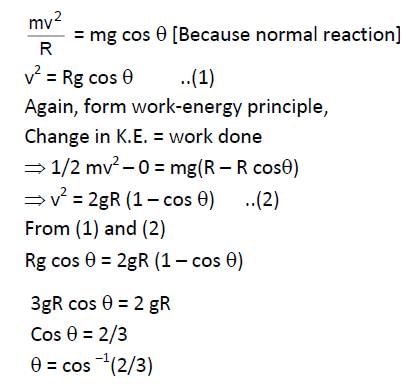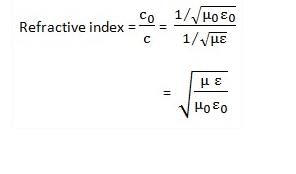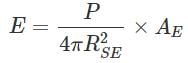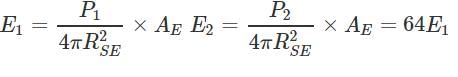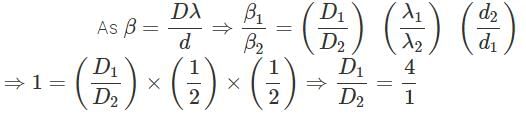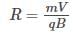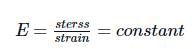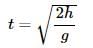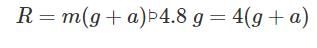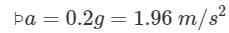JIPMER Full Mock Test - 1 - NEET MCQ
30 Questions MCQ Test JIPMER: Subject wise Tests & Practice Mock Tests 2024 - JIPMER Full Mock Test - 1
The frequency of an alternating voltage is 50 cycles/sec and its amplitude is 120V. Then the r.m.s. value of voltage is
The X - ray wavelength of L α line of platinum (Z - 78) is 1.30 Å . The X-ray wave length of L α line of molybdenum (Z = 42)
In a circuit two or more cells of the same e.m.f. are connected in parallel in order to
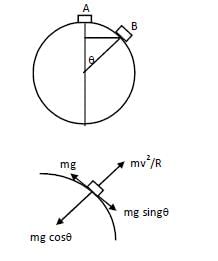
A particle slides from rest from the top to down the outside smooth surface of a fixed sphere of radius r. It leaves the surface when the radius to the particle makes with the vertical an angle equal to—
The equivalent capacitance between a and b for the combination of capacitors shown in figure where all capacitances are in microfarad is
When a wire is stretched and its radius becomes r/2, then its resistance will be
A step- up transformer is used on a 120 V line to provide a potential difference of 2400 V. If the primary coil has 75 turns, the number of turns in the secondary coil is
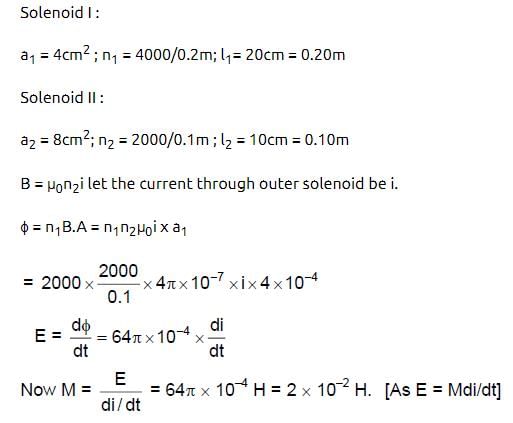
A solenoid of length 20 cm area of cross-section 4 cm2 and having 4000 turns is placed inside another solenoid of 2000 turns having a cross-section area 8 cm2 and length 10 cm. The mutual inductance of the system is
If ∈₀ and μ₀ represent the permittivity and permeability of vacuum and ∈ and μ represent the permittivity and permeability of medium, then refractive index of the medium is given by
Electric field and potential inside hollow charged conducting sphere are respectively where 'q' and 'r' are the charge and radius of given sphere.
When a satellite going round the eath in a circular orbit of radius r and speed V, loses some of its energy, then r and V change as
An electric dipole has the magnitude of its charge as q, and its dipole moment is p. It is placed in a uniform electric field E. If its dipole moment is along the direction of the field, then force acting on it and its potential energy are respectively
300 Joule of work is done in sliding a 2 kg. block on an inclined plane to a height of 10 meters. Taking value of acceleration due to gravity 'g' to be 10 m/s2, work done against friction is
If temperature of the sun were to increase from T to 2T and its radius from R to 2R, then how many times the radiant energy will be received on the earth?
A body of mass m is taken to the bottom of a deep mine. Then
In two separate setups of the Young's double slit experiment, fringe of equal width are observed when lights of wavelengths in the ratio 1:2 are used. If the ratio of the slit separation in two cases is 2:1, the ratio of distances, between the plane of slits and the screen, in the two setups is
Consider a 1 cc sample of air absolute temperature T₀ at sea level and another 1 cc sample of air at a height where the pressure is one-third atmosphere. The absolute temperature T of the sample at that height is
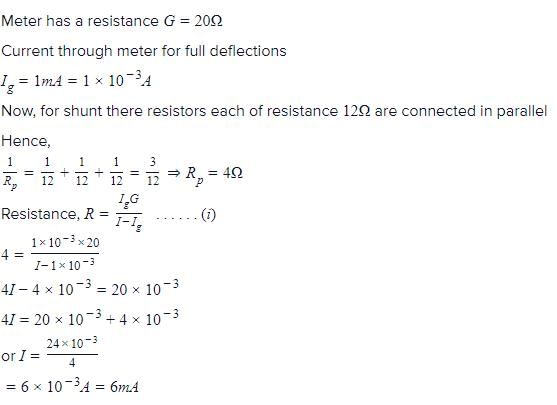
An electrical meter of internal resistance 20 Ω gives a full scale deflection when one milliampere current flows through it. The maximum current, that can be measured by using three resistors of resistance 12 Ω each, in milliamperes is
Which of the following particles will describe the smallest circle when projected with the same velocity perpendicular to the magnetic field ?
Work done in turning a magnet of magnetic moment M by an angle of 90º from the magnetic meridian is n times the corresponding work done to turn through an angle of 60º, where n is
If a bar magnet of moment μ is suspended in a uniform magnetic field B and it is given an angular deflection ' θ ' w.r.t its equilibrium position, the restoring torque on magnet is
A piece of steel floates in mercury. The specific gravities of mercury and steel are 13.6 and 7.8 respectively. For covering the whole piece, some water is poured over the mercury. What part of the steel piece will be inside the mercury?
According to the Hooke's law, if stress is increased, then ratio of stress to strain
A U-tube contains water and methylated spirit seperated by mercury. The mercury columns in the two arms are in level with 18 cm of water in one arm and 20 cm spirit in the other arm. The density of spirit is (Density of water=1 g-cm-3)
If an iron ball and a wooden ball of the same radius are released from a height 'h' in vaccum, then time taken by both of them to reach ground will be
A body of mass 4 kg weighs 4.8 kg when suspended in a moving lift. The acceleration of the lift is
|
4 videos|8 docs|51 tests
|
|
4 videos|8 docs|51 tests
|