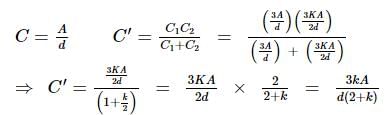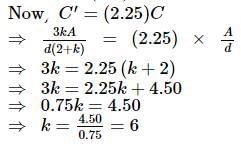JIPMER Full Mock Test - 4 - NEET MCQ
30 Questions MCQ Test JIPMER: Subject wise Tests & Practice Mock Tests 2025 - JIPMER Full Mock Test - 4
Voltage and current in an A.C. circuit are given by V = 5 sin(100πt - π/6) and I = 4sin(100πt + π/6)
An electron remains undeflected when passing perpendicular to mutually perpendicular electric and magnetic fields. If the magnetic field is 8 Gauss and elecric field is 4000 V/m, the velocity of the electron is
A parallel plate capacitor of plate area A and the plate separation d is charged to potential difference V and then the battery is disconnected. A slab of dielectric constant K is then inserted between the plates of the capacitor so as to fill the space between the plates. If Q, E and W denote respectively, the magnitude of charge on each plate, the electric field between the plates and work done by the system in question, in the process of inserting the slab, then select the incorrect relation from the following :
The average acceleration vector for a particle having a uniform circular motion is
Which of the following radiations have the least wavelength?
The length of a wire of a potentiometer is 100 cm, and the e.m.f. of its standard cell is E volt. It is employed to measure the e.m.f. of a battery, whose internal resistance is 0.5 Ω. If the balance point is obtained at l = 30 cm from the positive end, the e.m.f. of the battery is
In a circuit, n identical bulbs, each of power 'P' are joined in series across a power supply. The total power drawn by the bulbs is
A 12 Ω resistor and a 0.21 henry inductor are connected in series to an AC source operating at 20 volt, 50 cycle. The phase angle between the current and the source voltage is
A 220 V, 1000 watt bulb is connected across a 110 V mains supply. The power consumed will be
An infinite long plate has surface charge density σ . As shown in the figure a point charge q is moved from A to B. Net work done by electric field is:
A parallel plate air condenser has certain capacity. When 2/3 of the distance between the plates is filled with a dielectric, the capacity is found to increase by a factor of 2.25. The dielectric constant is
A brick of mass 2 kg beings to slide down on a plane inclined at an angle of 45º with the horizontal. The force of friction will be
If acceleration due to gravity on the earth's surface is g, then value of acceleration due to gravity at a height of 32 km above the earth's surface is
Two particles of eqaul mass go round a circle of radius R under the action of their mutual gravitational attration. The speed of each particle is
Mud houses are cooler in summer and warmer in winter because
In two separate setups of the Young's double slit experiment, fringe of equal width are observed when lights of wavelengths in the ratio 1:2 are used. If the ratio of the slit separation in two cases is 2:1, the ratio of distances, between the plane of slits and the screen, in the two setups is
Cooking gas containers are kept in a lorry moving with uniform speed. The temperature of the gas molecules inside will
An electrical meter of internal resistance 20 Ω gives a full scale deflection when one milliampere current flows through it. The maximum current, that can be measured by using three resistors of resistance 12 Ω each, in milliamperes is
The magnetic field of given length of wire for single turn coil at its centre is B, then its value for two turns coil for the same wire is
Cathode rays and canal rays produced in a certain discharge tube are deflected in the same direction, if
The null points are on the axial line of a bar magnet, when it is placed such that its south pole points
The material for which susceptibility remains constant for small values of magnetising field, increases for large values and then decreases for very large values is
A magnetised wire of magnetic moment M is bent into an arc of a circle that subtend and an angle of 60º at the centre. The equivalent magnetic moment is
An air bubble of radius 10 mm is rising at a steady rate of 8 mm-s⁻1 through a liquid of density 1.47x103 kg-m⁻3. Neglecting density of air, the coefficient of viscosity of the liquid is (Take g = 9.8 m-s2)
In a liquid flowing through a pipe, the pressure difference between its ends is made half and radius is doubled. If the initial rate of flow of liquid was Q, then new rate of flow of liquid through the pipe is
Two wires of the same material and length, but diameters in the ratio 1 : 2 are stretched by the same force. The elastic potential energy stored per unit volume for the two wires when stretched, will be in the ratio of
A constant torque of 1000 N-m turns a wheel of moment of inertia 200 kg-m2 about an axis through its centre. Its angular velocity after 3 sec is
A man is at rest in the middle of a pond on perfectly smooth ice. He can get himself to the shore by making use of Newton's
A constant acts on a body of mass 0.9 kg at rest for 10 s. If the body moves a distance of 250 m, the magnitude of the force is
Two lithium nuclei in a lithium vapour at room temperature do not combine to form a carbon nucleus because
|
4 videos|8 docs|51 tests
|
|
4 videos|8 docs|51 tests
|