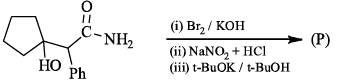IIT JAM Chemistry - MCQ Test 3 - Chemistry MCQ
30 Questions MCQ Test Mock Test Series for IIT JAM Chemistry - IIT JAM Chemistry - MCQ Test 3
Which of the following molecules will have the highest zero point vibrational energy:
Which one of the normal modes of ethylene is active in the infrared:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The moment of inertia for HCl gas can be determined from its microwave spectrum, which property of the HCl molecule may be obtained from the moment of inertia:
Overtones are observed in the vibrational spectra of diatomic molecules when:
The correct order of the fundamental vibrational frequency of the following diatomic molecules is:
The reaction of the bromo compound shown below with sodium ethoxide gives predominantly
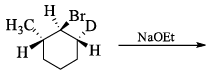
The reaction of l-bromo-2-fluorobenzene with furan in the presence of one equivalent to Mg gives:
The microwave spectrum of a molecule yields three rotational constants. The molecule is:
Electronegativity and Electron affinity of an element A are X and Y resp. Hence Ionisation potential is
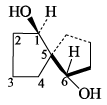
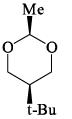
Which of the following is the most stable conformer of the following molecule:
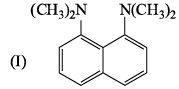
The increasing order of basicity among the following is :
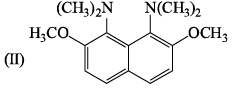

The major product expected from the following reaction is? 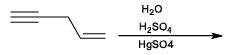
The population (N) distribution over states (n) of a diatomic molecular corresponds to:

Arrange the following in increasing order of Ka value:

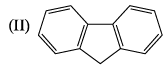
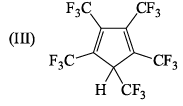
Correct energy profile for amine inversion and hybridization of nitrogen in transition state is:

Resonance frequencies for EPR and NMR are respectively in the spectral region:
Consider the cell Cd(s)/Cd+2(1.0M)// C+2(1.0M)/Cu(s). if we wanted to make a cell with a more positive voltage using the same substances, we should:
EMF of the following cell is 0.67 V at 298 K. Pt|H2(latm) |H+(pH = X) || KCl (IN) | Hg2Cl2(s) | Hg Calculate the pH of anode compartment. Given 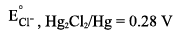
Rate of formation of SO3 in the following reaction
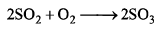
is 100g min-1. Hence, rate of disappearance of O2 is:
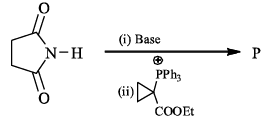
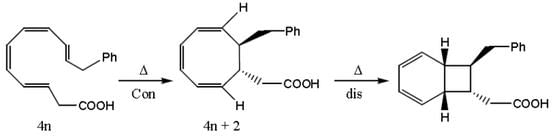
In the following sequence of pericyclic reactions X and Y are
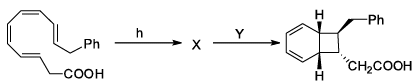
Specifi heat of N2 at constant pressure is 0.25 cal/g°C. Hence specific heat at constant volume is
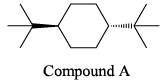
Bridge-head hydrogen of the conform of cis-decalin is positioned as:
The resonance energy of anthracene (from the below data)
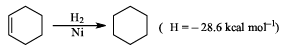
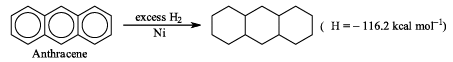
Given 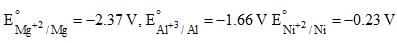 which of the following statement is incorrect:
which of the following statement is incorrect:
Weight of 14C have radioactivity 1 Curie (Disintegration constant is 4.4 × 10–12 sec-1 is ‘(in Kg)
In the reaction, P + Q → R + S the time taken for 75% reaction of P is twice the time taken for 50% reaction of P. The concentration of Q varies with reaction time as shown in the figure. The overall order of the reaction is:

|
2 docs|25 tests
|