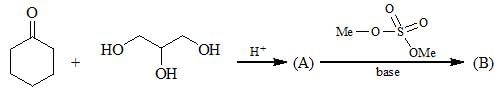IIT JAM Chemistry Mock Test 4 - Chemistry MCQ
30 Questions MCQ Test Mock Test Series for IIT JAM Chemistry - IIT JAM Chemistry Mock Test 4
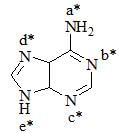
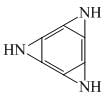
Favorable sites for protonation in following compound is/are:

The coordination number of Th in K4[Th (Ox)4(OH)2] is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The standard electrode potential E° and its temperature coefficient (dE/dT)P for a cell are 2V and –5 × 10–4 VK–1 at 300 K respectively. The cell emf would be?
Zn(s) + Cu2+ (aq) → Zn+2 (aq.) + Cu(s)
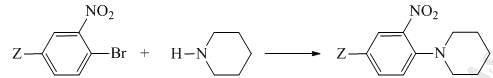
The suitable reagent for the following conversion is:
What would be the correct order of PI values for given amino acids?
(1) Lysine (2) Aspartic acid (3) Valine
Correct order of reactivity of following compounds in Dies-Alder reaction would be?
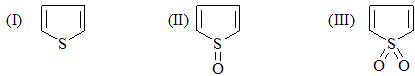
Benzoic acid (C6H5COOH) dimerizes in benzene. 'W' g of acids dissolved in 30 g of benzene shows a depression in freezing point equal to 2K. If the percentage association of the acid to form dimer is 80, then,numerical value of ‘W’ is [Kf = 5 Kg mol–1, Molar Mass = 122 mol–1]:
Correct formula of chromite is (It is an oxide mineral belonging to the spinel group):
The correct form of moment of inertia of CO2 molecules is:
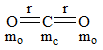
The reaction, MgO(s) + C(s) → Mg(s) + CO(g) for which ΔrHº=491.1KJ/mol-1and ΔrSº=198.0 KJ/mol-1is not feasible at 298 K. Temperature above which reaction will be feasible is?
Calculate the projection around z-axis forl = 2 and ml = 2:
The bond angle between two hybrid orbitals is 105°. The percentage s-character of hybrid orbital is between:
When S in the form of S8 is heated at 900 K, the initial pressure of 1 atm falls by 29% at equilibrium. This is because of conversion of S8 to S2. Find the value of equilibrium constant of this reaction.
0.2 M NaOH is titrated with 0.2 M, 20 ml, HA (Monobasic acid) till the equivalence point reached degree of dissociation of HA is supposed to be legible. Calculate the pH of the resulting solution at the end point. [Given Ka = 1.8 × 10–5]
The compound formed is/are in the positive test for nitrogen with the Lassaigne extract of an organic compound:
Determine enthalpy change (in kJ) for C3H8(g) +H2 (g) →C2H6(g)+CH4(g) at 25°C using heat of combustion value under standard conditions. Given: ΔHformC3H8(g) = –103.8 kJ/mole.

Which of the following represents the correct order of increasing first ionization enthalpy for Ca, Ba, S, Se, and Ar?
The reaction between [PdCl4]2– and C2H4 produces a new compound. Compared to free C2H4, the C—C bond order of the product is:
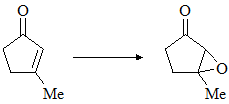
Consider the following chemical reaction and choose the correct product:
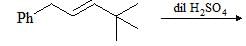
Consider the following chemical reaction and choose the correct alternative:
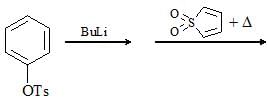 ; Final product is:
; Final product is:
Consider the following chemical reaction and choose major product:
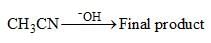
A ketone on treatment with bromine in methanol gives the corresponding Monobromo compound [X] having molecular formula C5H9BrO. The compound [X] when treated with Sodium methoxide in methanol produces [Y] as the major product, the 1H NMR spectrum data for compound [X] are: 1H NMR:δ 1.17 (d, 6H), 3.02 (m, 1H), 4.10 (s, 2H). The compound X and Y, respectively, are:
The correct order of the rate constant for the following series of reaction (Where Z = CF3/CH3/OCH3) is:
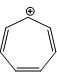
Which one of the following statements is true?
1. 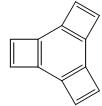 2.
2. 
3. 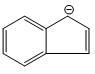 4.
4. 
Van Arkel method of purification of metals involves converting the metal to:
The adsorption of a gas is described by Langmuir Isotherm with equation constant, K = 0.5 K Pa–1 at 25°C. The pressure (in PK) when translational reaction coverage is 0.25 is:
A solution containing 4.5 mM of Cr2O72– and 15 mM of Cr3+ shows a pH of 2.0. Calculate the potential of half reaction. (E° of Cr2Cr72-→Cr3+ is 1.33 V)
|
2 docs|25 tests
|