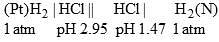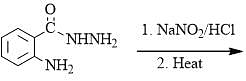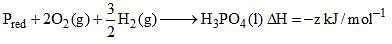IIT JAM Chemistry Mock Test 5 - Chemistry MCQ
30 Questions MCQ Test Mock Test Series for IIT JAM Chemistry - IIT JAM Chemistry Mock Test 5
Correct order of bond length of p, q, r, x and y given in following compound is:
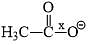
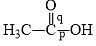
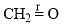
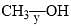
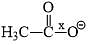
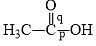
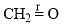
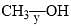
The correct order of the fundamental vibrational frequency of the following diatomic molecules is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The major product formed in the following reaction is:
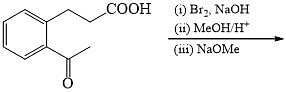
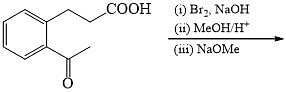
The pH of the blood is maintained by the balance between H2CO3 and NaHCO3. If the amount of CO2 in the blood is increased how will it affect the pH of blood?
The change in the hybrid state of BeCl2 in the solid state from vapour state is:
Which emission results in formation of an isotope of the parent element:
The major product formed in the following reaction is:

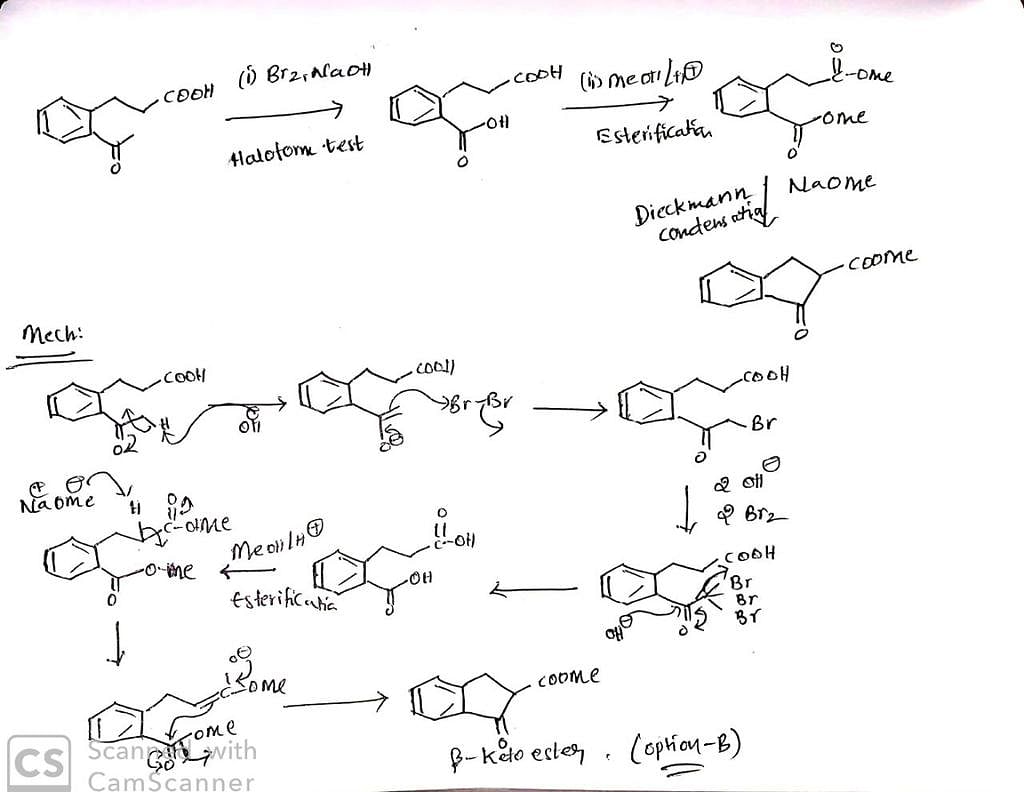
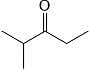
Which sequence of steps describes the best synthesis of 2-methyl-3-pentanone?
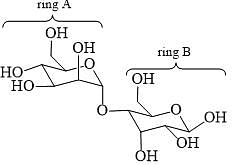
Which statements are correct about the following disaccharide?
The angular part of the wave function for the electron in a hydrogen atom is proportional to sin2θcosθe2iφ. The values of the azimuthal quantum number (l) and the magnetic quantum number (m) are, respectively
Which of the following reaction has abnormal forward rate of reaction:
Arrange the following in increasing order of C—O stretching frequency:
I. [Mn(CO)6]+ II. CO III. AlCl3(CO) IV.[V(CO)6]–
Which of the following is true about Phosphorus are?
Equivalent conductance of BaCl2, H2SO4 and HCl are x1, x2 and x3 S cm2 equiv–1 at infinite dilution. If specific conductance of saturated BaSO4 solution is y S cm–1 then Ksp of BaSO4 is:
0.004 M Na2SO4 is isotonic with 0.01 M Glucose. It’s degree of dissociation will be:
Molecules formula of a compound is C9H10O2. It shows following value in IR &1H NMR spectroscopy: IR (cm–1): 1745, 1225, 749, 697. 1H NMR: 2.06 (3H, s), 5.08 (2H, s), 7.22 (5H, s).
An organic compound A(C8H16O2) on treatment with an excess of Methylmagnesium chloride generated two alcohols B and C, whereas reaction of A with lithium Aluminium hydride generated only a single alcohol C. Compound B on treatment with an acid yielded an olefin ?(C6H12), which exhibited only a singlet at δ 1.6 ppm in the 1H NMR spectrum. The compound A is:
The major product formed in the following reaction is:

The cell reaction for the given cell is spontaneous if
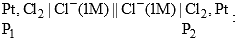
A metal crystallizes in two cubic phases, fcc and bcc whose unit cell lengths are 3.5Å and 3.0Å resp. What would be the ratio of their densities of fcc to bcc?
The species which by definition has zero standard molar enthalpy of formation at 298 K is:
Choose the correct statement(s) in reference to simple Harmonic and Anharmonic oscillator model?
(I) Two curves differ widely in lower and higher inter-nuclear distance region
(II) The actual energy diagram rises more steeply than harmonic parabola energy diagram in lower inter-nuclear distance region
(III) In the region near to equilibrium inter-nuclear distance, the curves do not show appreciable departure
(IV) In higher inter-nuclear distance region, rise for actual energy diagram is less steep
|
2 docs|25 tests
|