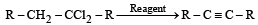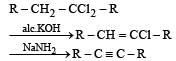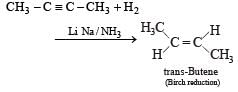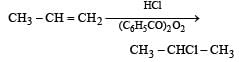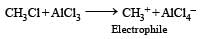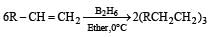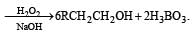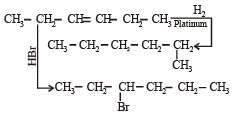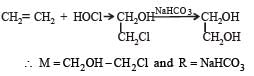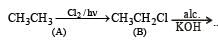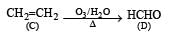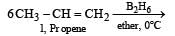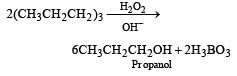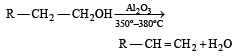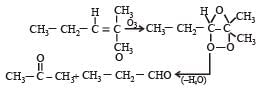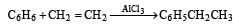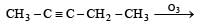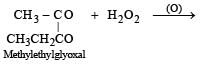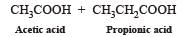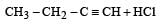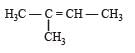31 Year NEET Previous Year Questions: Hydrocarbons - NEET MCQ
30 Questions MCQ Test Chemistry Class 11 - 31 Year NEET Previous Year Questions: Hydrocarbons
Acetylenic hydrogens are acidic because [1989]
Which is the most suitable reagent among the following to distinguish compound (3) from rest of the compounds ? [1989]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
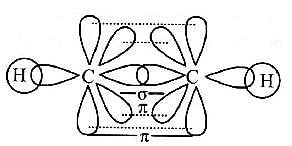
Select the true statement about benzene amongst the following [1992]
Reduction of 2-butyne with sodium in liquid ammonia gives predominantly : [1993]
A compound is treated with NaNH2 to give sodium salt. Identify the compound [1993]
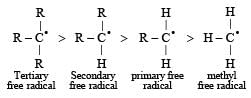
Reactivity of hydrogen atoms attached to different carbon atoms in alkanes has the order [1993]
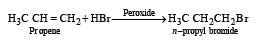
When hydrochloric acid gas is treated with propene in presence of benzoyl peroxide, it gives [1993]
Which of the following compounds has the lowest boiling point ? [1994]
When CH3Cl and AlCl3 are used in Friedel-Crafts reaction, the electrophile is [1994]
In the free radical chlorination of methane, the chain initiating step involves the formation of
The alkene R – CH = CH2 reacts readily with B2H6 and formed the product B which on oxidation with alkaline hydrogen peroxides produces [1995]
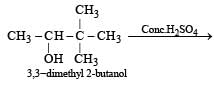
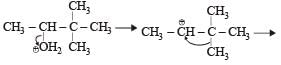
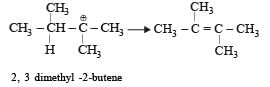
When 3, 3-dimethyl 2-butanol is heated with H2SO4, the major product obtained is [1995]
In the presence of platinum catalyst, hydrocarbon A adds hydrogen to form n-hexane. When hydrogen bromide is added to A instead of hydrogen, only a single bromo compound is formed. Which of the following is A? [1996]
In reaction sequence [1997]
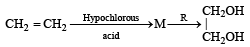
molecule 'M' and reagent 'R' respectively are
Which one of the following reactions is expected to readily give a hydrocarbon product in good yields ? [1997]
In commercial gas olines the type of hydrocarbons which are more desirable, is
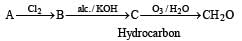
A hydrocarbon A on chlorination gives B which on heating with alcoholic potassium hydroxide changes into another hydrocarbon C. The latter decolourises Baeyer's reagent and on ozonolysis forms formaldehyde only. A is [1998]
The reaction of ethyl magnesium bromide with water would give [1999]
Which of the following reagents convert propene to 1-propanol? [2000]
For the formation of toluene by Friedal Craft reaction, reactants used in presence of anhydrous AlCl3 are [2000]
In preparation of alkene from alcohol using Al2O3 which is effective factor? [2001]
Which alkene on ozonolysis gives CH3CH2CHO and 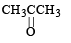
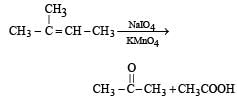
The compound [2003]
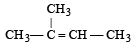
on reaction with NaIO4 in the presence of KMnO4 gives
Reaction of HBr with propene in the presence of peroxide gives [2004]
Using anhydrous AlCl3 as catalyst, which one of the following reactions produces ethylbenzene (PhEt)?[2004]
Which one of the following alkenes will react faster with H2 under catalytic hydrogenation conditions? [2005] (R = Alkyl Substituent)
Products of the following reaction : [2005]
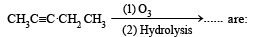
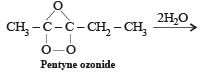
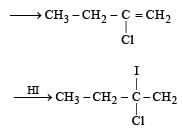
Predict the product C obtained in the following reaction of butyne-1. [2007]
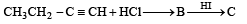
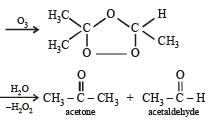
Which of the compounds with molecular formula C5H10 yields acetone on ozonolysis? [2007]
|
129 videos|244 docs|88 tests
|