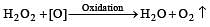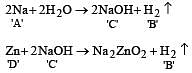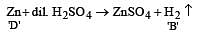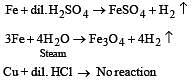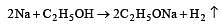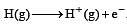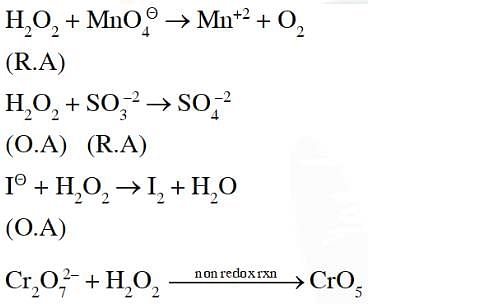31 Year NEET Previous Year Questions: Hydrogen (Old NCERT) - NEET MCQ
15 Questions MCQ Test Chemistry 31 Years NEET Chapterwise Solved Papers - 31 Year NEET Previous Year Questions: Hydrogen (Old NCERT)
Tritium, a radioactive isotope of hydrogen, emits which of the following particles? [2021]
Some statements about heavy water are given below:
(a) Heavy water is used as a moderator in nuclear reactors.
(b) Heavy water is more associated than ordinary water.
(c) Heavy water is more effective solvent than ordinary water.
Which of the above statements are correct? [2010]
(a) Heavy water is used as a moderator in nuclear reactors.
(b) Heavy water is more associated than ordinary water.
(c) Heavy water is more effective solvent than ordinary water.
Which of the above statements are correct? [2010]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
When H2O2 is oxidised the product is [1999]
When a substance A reacts with water it produces a combustible gas B and a solution of substance C in water. When another substance D reacts with this solution of C, it also produces the same gas B on warming but D can produce gas B on reaction with dilute sulphuric acid at room temperature. A imparts a deep golden yellow colour to a smokeless flame of Bunsen burner. A, B, C and D respectively are [1998]
Which one of the following pairs of substances on reaction will not evolve H2 gas? [1998]
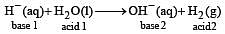
The hydride ion, H–, is a stronger base than the hydroxide ion, OH–. Which one of the following reactions will occur if sodium hydride (NaH) is dissolved in water? [1997]
The volume strength of 1.5 NH2O2 solution is
Which of the following groups of ions makes the water hard ? [1994]
The O – O – H bond angle in H2O2 is [1994]
The dielectric constant of H2O is 80. The electrostatic force of attraction between Na+ and Cl– will be [1994]
At its melting point ice is lighter than water because [1992]
The ionization of hydrogen atom would give rise to[1990]
Calgon used as a water softener is [1989]
Hydrogen peroxide acts both as an oxidising and as a reducing agent depending upon the nature of the reacting species. In which of the following cases H2O2 acts as a reducing agent in acid medium ? [1989]
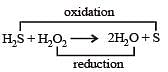
The reaction of H2O2 with H2S is an example of ........reaction
[1988]
|
29 docs|49 tests
|
|
29 docs|49 tests
|