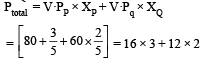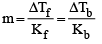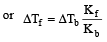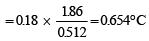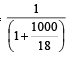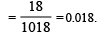31 Years NEET Previous Year Questions: Solutions - 2 - NEET MCQ
30 Questions MCQ Test Chemistry Class 12 - 31 Years NEET Previous Year Questions: Solutions - 2
An ideal solution is formed when its components [1988]
All form ideal solution except [1988]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The relative lowering of the vapour pressure is equal to the ratio between the number of [1991]
Which of the following aqueous solution has minimum freezing point ? [1991]
Blood cells retain their normal shape in solution which are [1991]
Which of the following modes of expressing concentration is independent of temperature ? [1992,1995]
Which one is a colligative property ? [1992]
If 0.1 M solution of glucose and 0.1 M solution of urea are placed on two sides of the semipermeable membrane to equal heights, then it will be correct to say that [1992]
At 25°C, the highest osmotic pressure is exhibited by 0.1 M solution of [1994]
Which one of the following salts will have the same value of van’t Hoff factor (i) as that of K4[Fe (CN)6]. [1994]
The number of moles of oxygen in one litre of air containing 21% oxygen by volume, in standard conditions, is [1995]
Vapour pressure of benzene at 30°C is 121.8 mm.When 15 g of a non volatile solute is dissolved in 250 g of benzene its vapour pressure decreased to 120.2 mm. The molecular weight of the solute (Mo. wt. of solvent = 78) [1995]
According to Raoult's law, relative lowering of vapour pressure for a solution is equal to [1995
The vapour pressure at a given temperature of an ideal solution containing 0.2 mol of a nonvolatile solute and 0.8 mol of solvent is 60 mm of Hg. The vapour pressure of the pure solvent at the same temperature is [1996]
Which of the following 0.10 m aqueous solutions will have the lowest freezing point ? [1997]
A 5% solution of cane sugar (mol. wt. =342) is isotonic with 1% solution of a substance X. The molecular weight of X is [1998]
The vapour pressure of a solvent decreased by 10mm of mercury when a non-volatile solute was added to the solvent. The mole fraction of the solute in the solution is 0.2. What should be the mole fraction of the solvent if the decrease in the vapour pressure is to be 20mm of mercury?
Which of the following statements, regarding the mole fraction (x) of a component in solution, is incorrect? [1999]
Which of the following colligative property can provide molar mass of proteins (or polymers or colloids) with greatest precision ? [2000]
The beans are cooked earlier in pressure cooker, because [2001]
Molarity of liquid HCl will be, if density of solution is 1.17 gm/cc [2001]
1 M, 2.5 litre NaOH solution is mixed with another 0.5 M, 3 litre NaOH solution. Then find out the molarity of resultant solution [2002]
A solution con tains non -volatile solute of molecular mass M2. Which of the following can be used to calculate the molecular mass of solute in terms of osmotic pressure ? [2002]
A solution containing components A and B follows Raoult's law when [2002]
Formation of a solution from two components can be considered as [2003]
(i) Pure solvent → separated solvent molecules, ΔH1
(ii) Pure solute → separated solute molecules, ΔH2
(iii) Separted solvent & solute molecules → Solution, ΔH3
Solution so formed will be ideal if
(a) ΔHsoln = ΔH1 + ΔH2 - ΔH3
(b) ΔHsoln = ΔH1 + ΔH2 + ΔH3
(c) ΔHsoln = ΔH1 - ΔH2 - ΔH3
(d) ΔHsoln = ΔH3 - ΔH1 - ΔH2
Camphoris often used in molecular mass determination because [2004]
The vapour pressure of two liquids ‘P’ an d ‘Q’ are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be [2005]
A solution of urea (m ol. mass 56 g mol-1) boils at 100.18°C at the atmospheric pressure. If Kf and Kb for water are 1.86 and 0.512 K kg mol-1 respectively, the above solution will freeze at [2005]
The mole fraction of the solute in one molal aqueous solution is: [2 00 5]
A solution of acetone in ethanol [2006]
|
108 videos|286 docs|123 tests
|


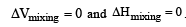
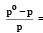 mole fraction of solute
mole fraction of solute 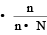

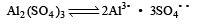
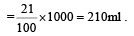
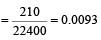
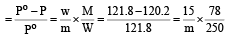
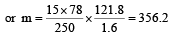
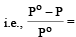 mole fraction of solute
mole fraction of solute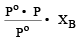
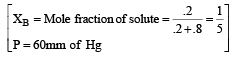
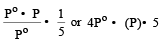
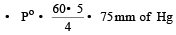
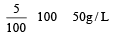
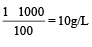
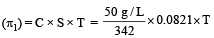
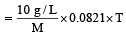
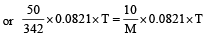
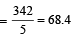
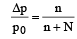 (mole fraction of solute)
(mole fraction of solute)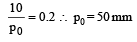
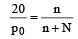 (Mole fraction of solute)
(Mole fraction of solute)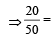 mole fraction of solute
mole fraction of solute
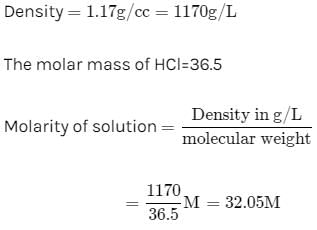
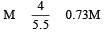
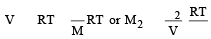
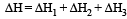 (Accroding to Hess's law) i.e., for ideal solutions there is no change in magnitude of the attractive forces in the two components present.
(Accroding to Hess's law) i.e., for ideal solutions there is no change in magnitude of the attractive forces in the two components present.