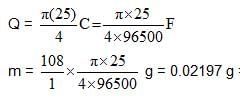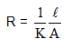Test: Electrochemistry - NEET MCQ
30 Questions MCQ Test NCERT Based Tests for NEET - Test: Electrochemistry
What is cell entropy change of the following cell?
Pt(s) | H2(g) | CH3COOH, HCl || KCl (aq) |Hg2Cl2| (s) | Hg
P = 1 atm 0.1M 0.1M
Emf of the cell is found to be 0.045 V at 298 K and temperature coefficient is
3.4 x10–4 VK–1
Given Ka (CH3COOH) = 10–5 M
Following cell has EMF 0.7995 V.
Pt | H2 (1 atm) | HNO3 (1M) || AgNO3 (1M) | Ag
If we add enough KCl to the Ag cell so that the final Cl- is 1M. Now the measured emf of the cell is 0.222 V.
The Ksp of AgCl would be :
The solubility of [Co(NH3)4Cl2] CIO4_________ if the = 50,
= 70, and the measured resistance was 33.5Ω in a cell with cell constant of 0.20 is ____.
We have taken a saturated solution of AgBr.Ksp of AgBr is 12 x 10 – 14 . If 10 – 7 mole of AgNO3 are added to 1 litre of this solution then the conductivity of this solution in terms of 10 – 7 Sm – 1 units will be
[given 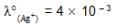 Sm2 mol-1
Sm2 mol-1 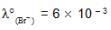 Sm2 mol-1, 5 x 10-3 Sm2mol-1]
Sm2 mol-1, 5 x 10-3 Sm2mol-1]
At 298K the standard free energy of formation of H2O(L) is – 237.20kJ/mole while that of its ionisation into H+ ion and hydroxyl ions is 80 kJ/mole, then the emf of the following cell at 298 K will be
H2(g,1 bar) | H+ (1M) || OH– (1M) | O2 (g, 1bar)
Which of the following cell can produce more electric work.
A hydrogen electrodes is immersed in a solution with pH = 0 (HCl). By how much will the potential (reduction) change if an equivalent amount of NaOH is added to the solution. (Take PH2 = 1 atm) T = 298 K.
At what does the following cell have its reaction at equilibrium?
Ag(s) | Ag2CO3(s) | Na2CO3 (aq) || KBr(aq) | AgBr(s) | Ag(s)
KSP = 8 x 10 – 12 for Ag2CO3 and KSP = 4 x 10 – 13 for AgBr
Calculate the EMF of the cell at 298 K
Pt|H2(1atm)|NaOH(xM),NaCl(xM)|AgCl(s)|Ag
If E°cl-/AgCl/Ag = + 0.222 V
A current of 0.1A was passed for 2hr through a solution cuprocyanide and 0.3745g f copper was deposited on the cathode. Calculate the current efficiency for the copper deposition.
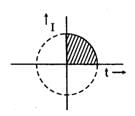
With t taken in seconds and I taken in Amp, the variation of I follows the equation
t2 + I2 = 25
what amount of Ag will be electrodeposited with this current flowing in the interval 0-5 second ? (Ag : 108)
A resistance of 50Ω is registered when two electrodes are suspended into a beaker containing a dilute solution of a strong electrolyte such that exactly half of the them are submerged into solution. If the solution is diluted by adding pure water (negligible conductivity) so as to just completely submerge the electrodes, the new resistance offered by the solution would be
The standard reduction potential of a silver chloride electrode is 0.2 V and that of a silver electrode is 0.79 V. The maximum amount of AgCl that can dissolve in 106 L of a 0.1 M AgNO3 solution is
Calculate the cell EMF in mV for
Pt|H2(1atm) |HCl(0.01M)|AgCl(s)| Ag(s) at 298 K
If ΔG°r values are at 25°C
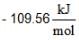 for AgCl(s) and
for AgCl(s) and 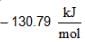 for H+ + Cl-) (aq)
for H+ + Cl-) (aq)
Adiponitrile is manufactured electrolytically from acrylonitrile
CH2 = CHCN → CN – (CH2)4 – CN
How many kg of adiponitrile (molecular mass = 108) is produced in 9.65 hr using a current of 3750 A with 80% efficiency
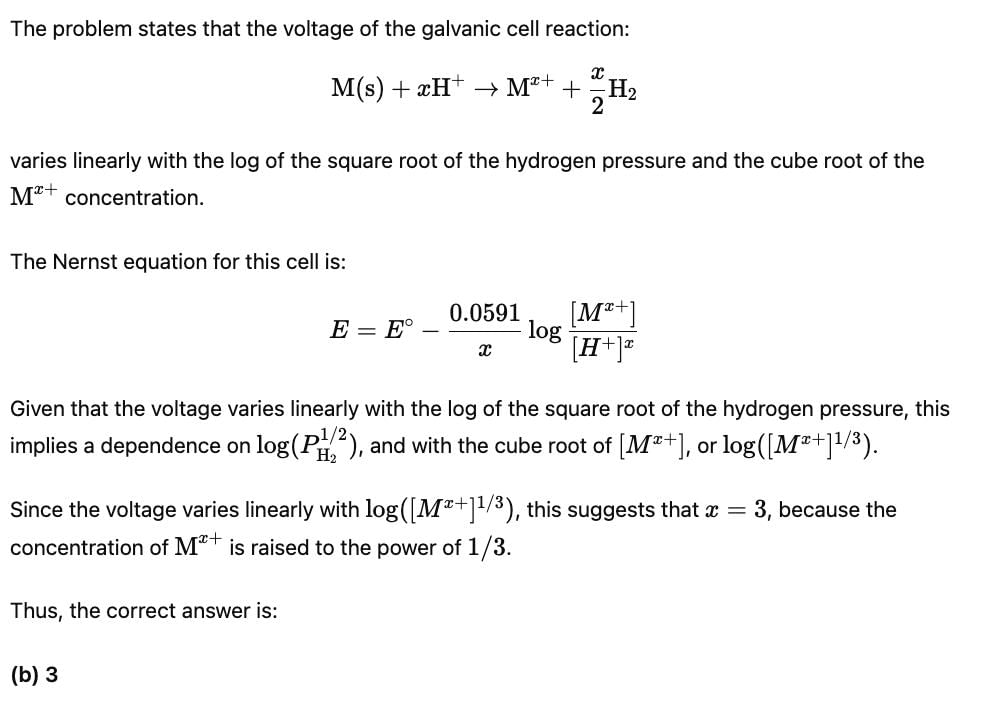
It is observed that the voltage of a galvanic cell using the reaction M(s) + xH+→ Mx+ + X/2H2 varies linearly with the log of the square root of the hydrogen pressure and the cube root of the Mx+ concentration. The value of x is
Acetic acid has Ka = 1.8 X 10 – 5 while formic acid had Ka = 2.1 X 10–4. What would be the magnitude of the emf of the cell
Consider the cell Ag(s)|AgBr(s)|Br-(aq)||AgCl(s)|Cl-(aq)|Ag(s) at 25°C. The solubility product constants of AgBr & AgCl are respectively 5 X 10 – 13 & 1 X 10 – 10. For what ratio of the concentrations of Br- & Cl- ions would the emf of the cell be zero ?
Value of for SrCl2 in water at 25°C from the following data:

Calculate the useful work of the reaction Ag(s) + 1/2Cl2(g) → AgCl(s)
Given E°cl2/cl- = + 1.36 V, E°AgCl/Ag,Cl- = 0.22 V
If Pcl2 = 1 atm and T = 298 K
Which of these ions Cu+, Co3+, Fe2+ is stable in aqueous medium.
Given :
E°Cu2+/Cu+ = 0.15 volt ; E°Cu+/Cu = 0.53 V ; E°Co3+/Co2+ = 1.82 V ;
E°Fe3+/Fe2+ = 0.77 V ; E°Fe2+Fe = - 0.44 V ; E°O2,H+/H2O = 1.23 V
Select the correct statement if -
E°Mg2+/Mg = - 2.4V, E°Sn4+/Sn2+ = 0.1 V, E°MnO4-,H+/Mn2+ = 1.5 V, E° I2/I- = 0.5 V Here,
What is the value of pKb (CH3COOH-) if λm∞ = 390 & λm = 7.8 for 0.04 of a CH3COOH at 25°C
The temperature coefficient of a standard Cd-cell is – 5.0X10– 5 Vk– 1 whose emf at 25°C is 1.018 V. During the cell operation, the temperature will -
A cell Ag | Ag+ || Cu++ | Cu initially contains 2M Ag+ and 2M Cu++ ions. The charger in cell potential after the passage of 10 amp current for 4825 sec is:
For the cell (at 298 K)
Ag(s) | AgCl(s) | Cl-(aq) || AgNO3(aq) | Ag(s)
Which of the following is correct –
During an electrolysis of conc. H2SO4, perdissulphuric acid (H2S2O8) and O2 from in equimolar amount. The amount of H2 that will form simultaneously will be (2H2SO4 → H2S2O8 + 2H+ + 2e-)
Statement-1: In electrochemical cell, we cannot use KCI in the salt bridge if anodic or cathodic compartment consists of Ag+ of Pb2+ ion.
Statement-2: Salt bridge is employed to maintain the electrical neutrality and to minimize the liquid-liquid junction potential.
Statement-1: Zinc protect the iron better than tin even after it cracks.
Statement-2: E°OPzn < E°OPfe But E°OPSn > E°OPfe
|
684 tests
|


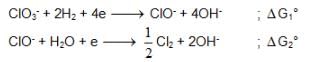
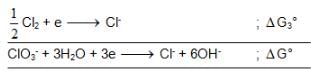
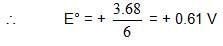
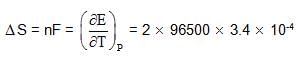

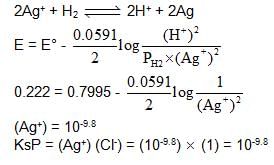
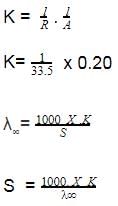
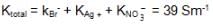
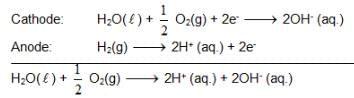 also we have
also we have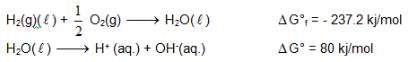 Hence for cell reaction
Hence for cell reaction