Test: Dimensions of Unit Cells (Old NCERT) - NEET MCQ
10 Questions MCQ Test Chemistry Class 12 - Test: Dimensions of Unit Cells (Old NCERT)
If an element crystallizes as a simple cube, what is the volume of an element provided its density is 1.5 g/cm3 and atomic mass of the element is 63?
Mass of an atom in an unit cell is calculated by which of the following formula if M is the molar mass:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the formula to calculate the density of a unit cell 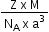 what is z ?
what is z ?
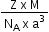 what is z ?
what is z ?Volume of a body centred cubic unit cell is
Density of a unit cell is equal to mass of unit cell divided by
The number of atoms (z) in simple cubic unit cell is
X-ray diffraction studies show that copper crystallizes in an fcc unit cell with cell edge of 3.608 x 10-8 cm. In a separate experiment, copper is determined to have a density of 8.92 g/cm3, calculate the atomic mass of copper.
An element with bcc geometry has atomic mass 50 and edge length 290 pm. The density of unit cell will be
The number of octahedral void(s) per atom present in a cubic close-packed structure is
|
100 videos|282 docs|123 tests
|