Test: Imperfections in Solids (Old NCERT) - NEET MCQ
15 Questions MCQ Test Chemistry Class 12 - Test: Imperfections in Solids (Old NCERT)
Which is the defect represented by the given figure


What is the effect of Frenkel defect on the density of ions solids?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Alkali halides do not show Frenkel defect because
What type of stoichiometric defect is shown by ZnS?
Which type of crystal defect is shown in the given figure?

In the given crystal structure what should be the cation X which replaces Na+ to create a cation vacancy?
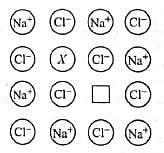
An electron trapped in an anion site in a crystal is called
In the following figure, the blank X is known as __________ and why?

The anionic sites occupied by unpaired electrons are called F-centres or colour centres. They impart (X) colour to the crystals of NaCl. Excess of lithium makes LiCl crystals (Y) and excess of potassium makes KCl crystals (Z). (X), (Y) and (Z) are
Zinc oxide loses oxygen on heating according to the reaction,

It becomes yellow on heating because
Which of the following will have metal deficiency defect?
Experimentally it was found that a metal oxide has formula M0.98O. Metal M, is present as M2+ and M3+ in its oxide. Fraction of the metal which exists as M3+ would be
Mark the incorrect pair from the following.
|
101 videos|287 docs|123 tests
|

















