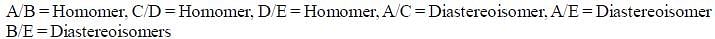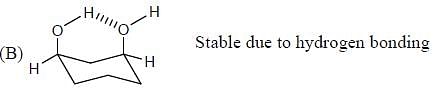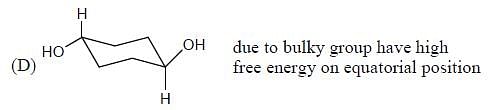Stereochemistry - GATE Chemistry MCQ
20 Questions MCQ Test GATE Chemistry Mock Test Series - Stereochemistry
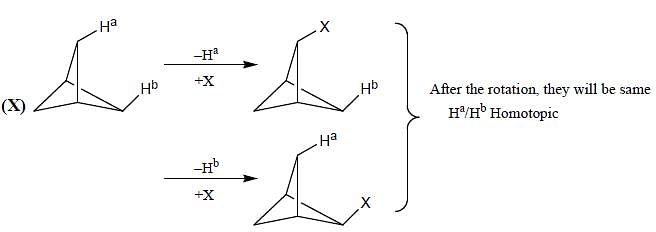
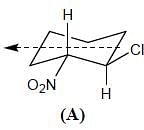
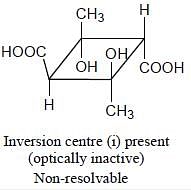
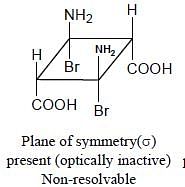
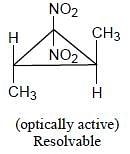
Topological relation between Ha and Hb in given molecules respectively.


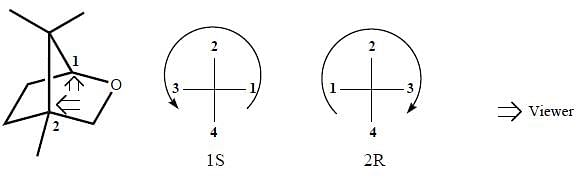
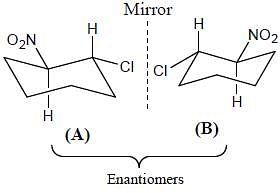
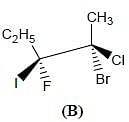
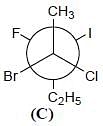
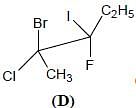
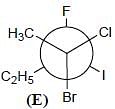
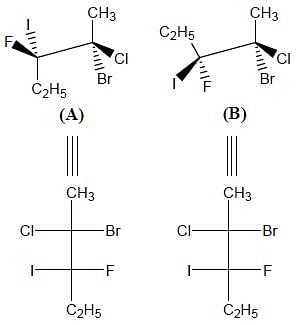
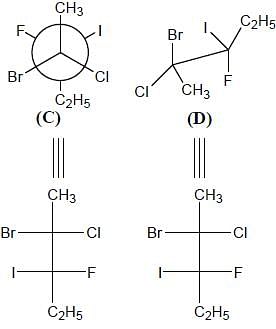
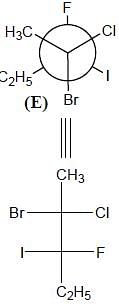
Correct configurations of stereo centres for the given molecules A and B respectively, will be.


| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
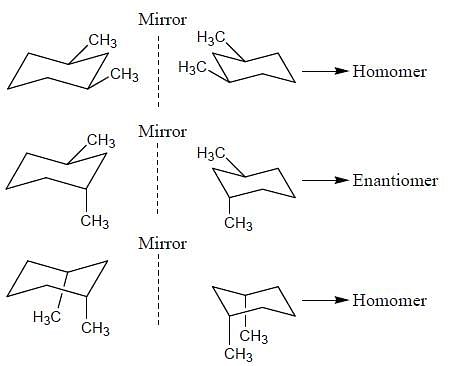
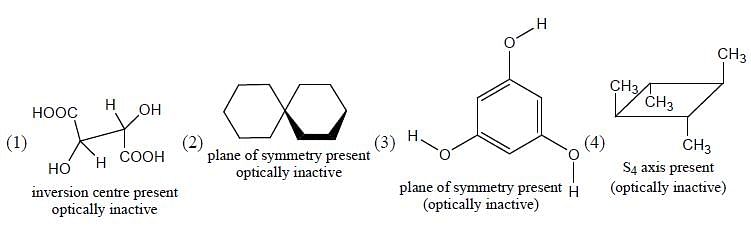
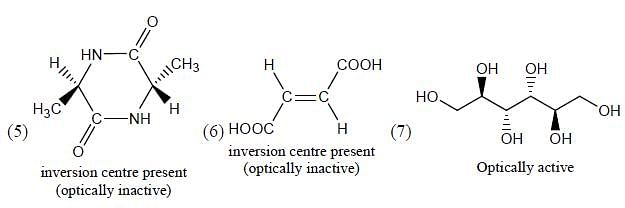
Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?
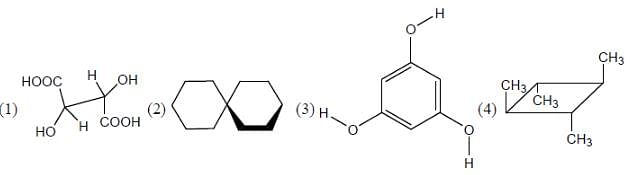
The total number of isomer possible for 1, 3-dimethylcyclohexane is/are_______
(+)-Mandelic acid has a specific rotation of +1600. The observed specific rotation of a mixture of 40%(-) mandelic acid and 60% (+)- mandelic acid is _______(answer should be an integer).
Which of the following of molecule can Not give conformational isomers?
The configuration (R/S notation) at C-1 and C-6 of the compound below are
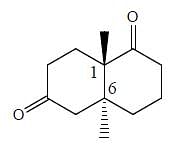
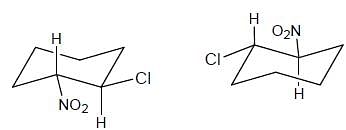
Which of the following statements can be deduced about the stereochemistry of this compound?
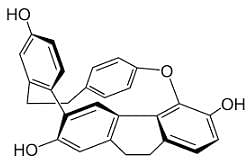
Conformation of some compounds are given below:
Out of these structures, less prefered conformation is
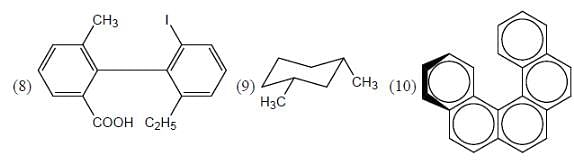
Which of the following is an alkane which can exhibit optical activity?
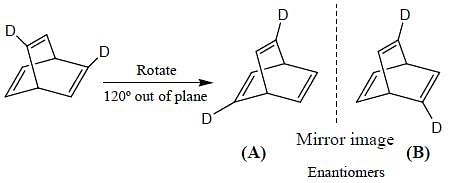
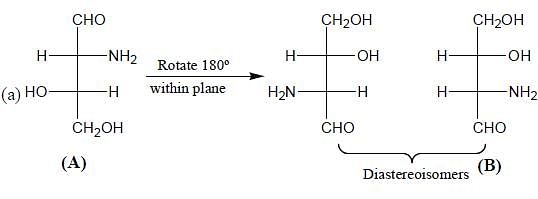
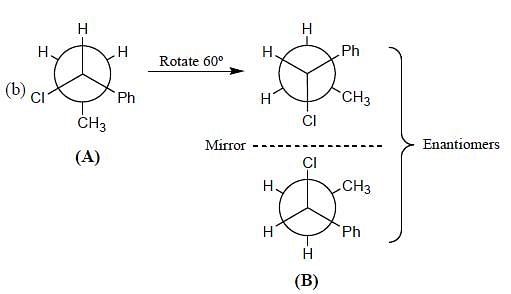
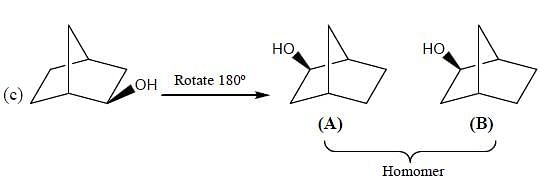
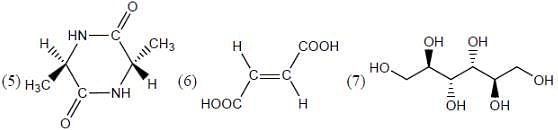
What is the stereochemical relationship between the following two molecules? 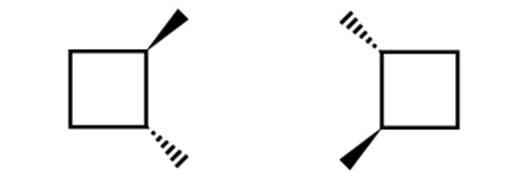
|
18 docs|37 tests
|
|
18 docs|37 tests
|