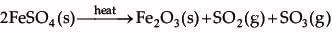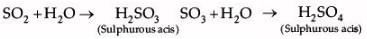Test: Class 10 Science: CBSE Sample Question Paper- Term I (2021-22) - Class 10 MCQ
30 Questions MCQ Test CBSE Sample Papers For Class 10 - Test: Class 10 Science: CBSE Sample Question Paper- Term I (2021-22)
Reema took 5ml of Lead Nitrate solution in a beaker and added approximately 4ml of Potassium Iodide solution to it. What would she observe?
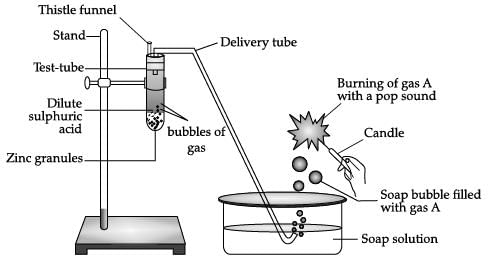
Identify gas A in the following experiment.

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following two combinations are correct?

Which of the following two combinations are correct?

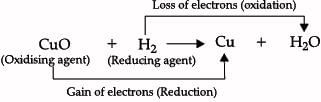
Which of the following correctly represents a balanced chemical equation?
The graph given below depicts a neutralization reaction (acid + alkali → salt + water).
The pH of a solution changes as we add excess of acid to an alkali.
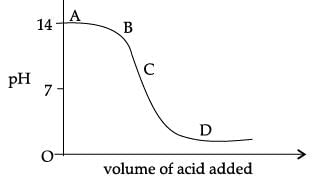
Which letter denotes the area of the graph where both acid and salt are present?
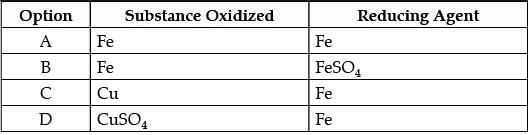
In the reaction of iron with copper sulphate solution:
CuSO4 + Fe → Cu + FeSO4
Which option in the given table correctly represents the substance oxidised and the reducing agent?
The chemical reaction between copper and oxygen can be categorized as:
Which of the given options correctly represents the Parent acid and base of Calcium Carbonate?
How will you protect yourself from the heat generated while diluting a concentrated acid?
Why is it important to balance a skeletal chemical equation?
Carefully study the diagram of the human respiratory system with labels A, B, C and D. Select the option which gives correct identification and main function and /or characteristic.
Identify the option that indicates the correct enzyme that is secreted in location A, B and C.
Opening and closing of stomatal pore depends on:
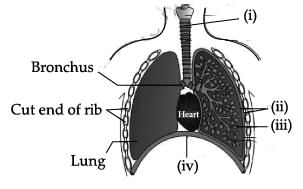
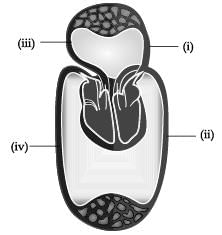
The figure given below shows a schematic plan of blood circulation in humans with labels (i) to (iv). Identify the correct label with its functions?
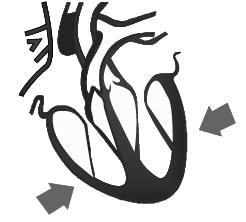
Identify the phase of circulation which is represented in the diagram of heart given below. Arrows indicate contraction of the chambers shown.
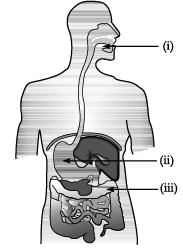
Observe the diagram of Human digestive system.
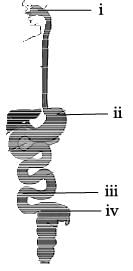
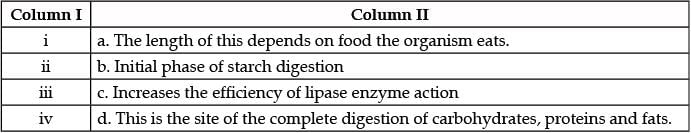
Match the labeling referred in column I and correlate with the function in column II.
Which of the following mirror is used by a dentist to examine a small cavity in a patient’s teeth?
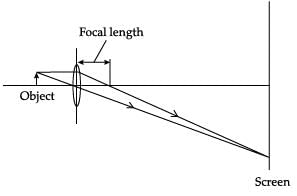
Which diagram shows image formation of an object on a screen by a converging lens?
Which of the following can make a parallel beam of light when light from a point source is incident on it?
Consider these indices of refraction: glass: 1.52; air: 1.0003; water: 1.333. Based on the refractive indices of three materials, arrange the speed of light through them in decreasing order.
If a beam of red light and a beam of violet light are incident at the same angle on the inclined surface of a prism from air medium and produce angles of refraction r and v respectively, which of the following is correct?
Examine the above figure and state which of the following option is correct? [one small box in the figure is equal to 1 cm]
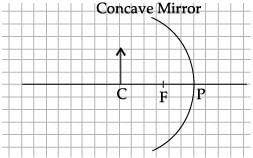
The angle of incidence from air to glass at the point O on the hemispherical glass slab is.

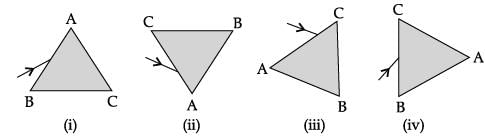
A prism ABC (with BC as base) is placed in different orientations. A narrow beam of white light is incident on the prism as shown in below Figure. In which of the following diagrams, after dispersion, the third colour from the top of the spectrum corresponds to the colour of the sky?
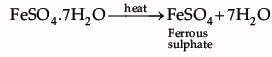
Shyam heated a small amount of light green colored compound X in a test tube. He found that the compound losed some water and then gas Z with suffocating smell comes out. The vapors of gas are collected and dissolved in water. The solution turns blue litmus red. The residue Y left in the test tube turns reddish brown.
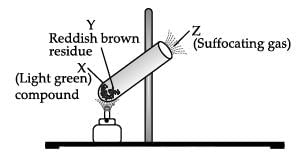
Identify X,Y and Z
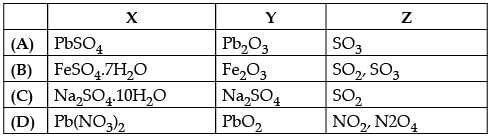
Choose the correct option .
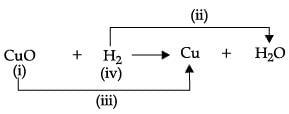
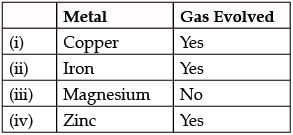
Study the given experimental set-up carefully. Based on the diagram, Choose the correct observation(s) ?
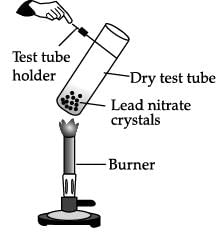
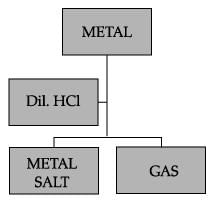
The given experimental set up is used to test a few solutions which contain hydrogen but are not categorized as acids.
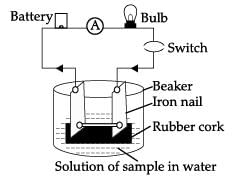
Which of these observation is incorrect?
An electrolytic cell consists of
(i) positively charged cathode
(ii) negatively charged anode
(iii) positively charged anode
(iv) negatively charged cathode
An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
|
303 docs|7 tests
|
|
303 docs|7 tests
|