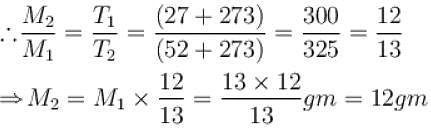Kinetic Theory Of Gases NAT Level - 2 - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Kinetic Theory Of Gases NAT Level - 2
Air is filled at 60°C in a vessel of open mouth. The vessel is heated to a temperature T so that 1/4th part of the air escapes. Assuming the volume of vessel remaining constant, the value of T is (in °C).
A gas at a certain volume and temperature has pressure 75cm of Hg. If the mass of the gas is doubled at the same volume and temperature, its new pressure in units of cm of Hg level?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A flask contains 10-3 m3 gas. At a temperature, the number of molecule of oxygen are 3 × 1022 . The mass of an oxygen molecule is 5.3 × 10-26 kg and at that temperature, the rms velocity of molecules is 400 m/s. The pressure (in 104 N/m2 ) of the gas in the flask is.
The root means square speed of hydrogen molecules at 300 K is 1930 m/s. Then the root mean square of oxygen molecules at 900 K will be (in m/s).
At which of the following temperature would the molecules of a gas have twice the average kinetic energy they have at 20°C (in °C)
The speeds of 5 molecules of a gas (in arbitrary units) are as follows : 2, 3, 4, 5, 6. The root mean square speed for these molecules is.
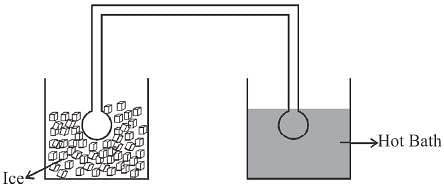
Two identical glass bulbs are inter connected by a thin glass tube. A gas is filled in these bulbs at NTP. If one bulb is placed in ice and another bulb is placed is hot bath, then the pressure of the gas becomes 1.5 times. the temperature of hot bath will be (in °C).
Hydrogen gas is filled in a balloon at 20°C. If temperature is made 40°C, pressure remaining same, what fraction of hydrogen will come out?
A closed vessel contains 8gm of oxygen and 7gm of nitrogen. The total pressure is 10atm at a given temperature. If now oxygen is absorbed by introducing a suitable absorbent the pressure of the remaining gas (in atm) will be?
A flask is filled with 13 gm of an ideal gas at 27°C and its temperature is raised to 52°C. The mass of the gas that has to be released to maintains the temperature of the gas in the flask at 52°C and the pressure remaining the same is (in gm).


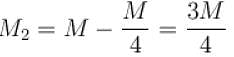 (as 1/4 th part of air escapes)
(as 1/4 th part of air escapes)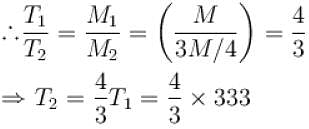
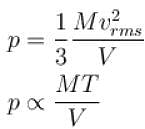
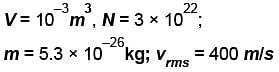
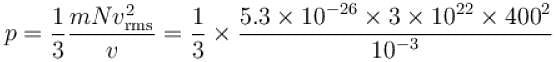
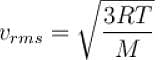
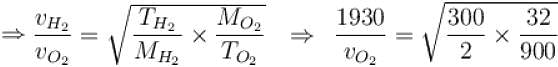
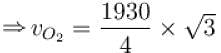
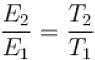
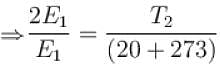
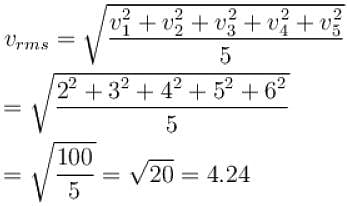
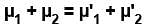
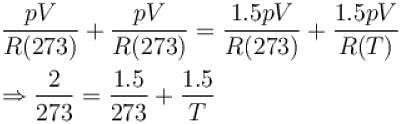
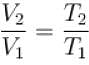
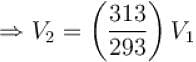
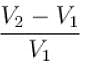
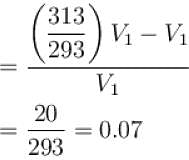
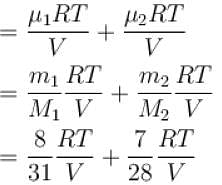
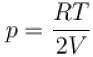 ...(i)
...(i)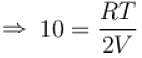
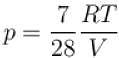 ...(ii)
...(ii)