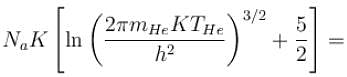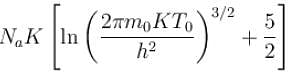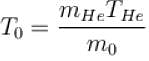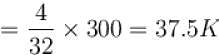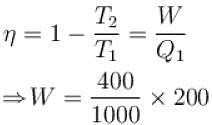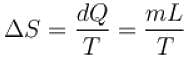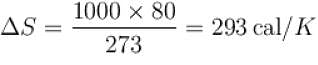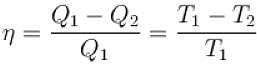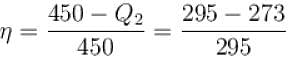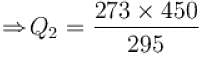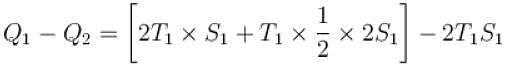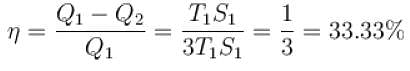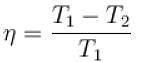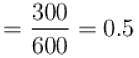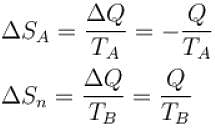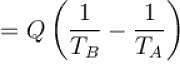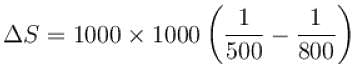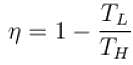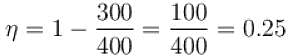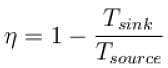Second Law Of Thermodynamics NAT Level - 1 - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Second Law Of Thermodynamics NAT Level - 1
Two ends of a rod are kept at 127ºC and 227ºC. When 2000 cal of heat flows in this rod, then change in entropy (in cal/K) is
One mole of helium and one mole of oxygen contained in two vessels. If temperature of Helium be 300K, what is the temperature of oxygen in Kelvin
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
An engine absorbs heat at temperature of 1000K and rejects heat at 600K. If the engine operates at maximum possible efficiency, the amount of work performed by the amount of work performed by the engine for 200J heat input is (in Joules)
1kg of ice melts at 0ºC into water at the same temperature. The change in entropy is (in cal/K) ____.
A heat pump working on the Carnot cycle maintains the inside temperature of a house at 22ºC by supplying 450 kJ/s. If the outside temperature is 0ºC, the heat taken, in kJ/s, from the outside air is approximately.
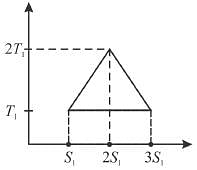
A reversible engine cycle is shown in the following T-S diagram. The efficiency of the engine (in percentage) is
The efficiency of a heat engine working between heat reservoirs at temperature 327ºC and 27ºC respectively (in percentage) is
An amount of heat 1000kJ is transferred from a heat reservoir at temperature TA to another heat reservoir at temperature TB . If TA = 800K and and TB = 500K, what is the change in entropy ΔS of the combined system in JK-1
A Carnot engine operating between 27ºC and 127ºC has efficiency equal to (in percentage).
For a reversible heat engine, the efficiency can be 100% if the temperature of the sink (in K) is


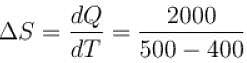
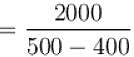
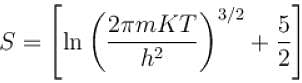 of a gas containing N particles is
of a gas containing N particles is