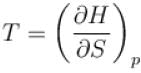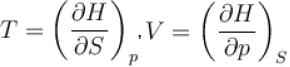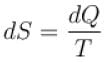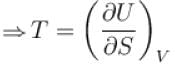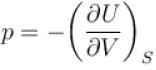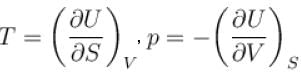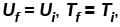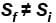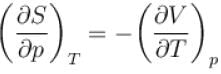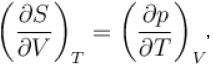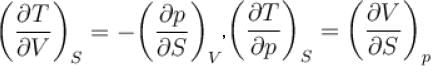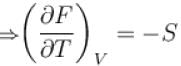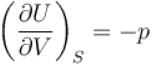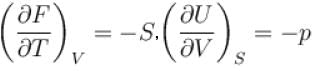Thermodynamic Potential MSQ - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - Thermodynamic Potential MSQ
For liquifying gas, it is essential that the gas
Select one or more:
Select one or more:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which among the following for Maxwell’s relation are correct?
(U - internal energy, H - enthalpy, S - entropy)
Select one or more:
According to consequences of the second law of thermodynamics (symbols have their usual meaning)
Select one or more:
Which of the following statements are true?
Select one or more:
Denoting temperature, entropy and internal energy by T, S and U respectively and their initial and final values by subscript i and f respectively, the Joule Thomson expansion of an ideal gas can be expressed as
Select one or more:
Which of the following are correct terms of Maxwell’s relation
Select one or more:
For an isolated thermodynamical system p, V, T, U, S and F represent the pressure, volume, temperature, internal energy,entropy and free energy respectively. Then, following relation are true
Select one or more:
For a thermodynamic system Helmholtz free energy is a function of
Select one or more: