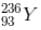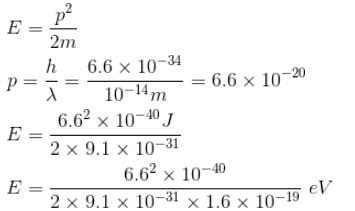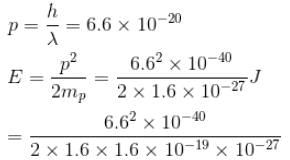Nuclear Physics MSQ - Physics MCQ
10 Questions MCQ Test - Nuclear Physics MSQ
For a radioactive substance with half life t1/2 and initial number of nuclei N0. The number the nuclei decayed in 3 half lives is :
A singly charged positive ion is accelerated through a potential difference of 1000V in a mass spectrograph. It then passes through a uniform magnetic field B = 1500 Gauss and then deflected into a circular path of radius 0.122 m.
Select the correct statements
A singly charged positive ion is accelerated through a potential difference of 1000V in a mass spectrograph. It then passes through a uniform magnetic field B = 1500 Gauss and then deflected into a circular path of radius 0.122 m. The mass number of the ion is :
1 mg of a radioactive material with half life 1600 years is kept for 2000 years. Choose the correct option.
What are the reasons leading to the discovery of a neutrino in the case of a Beta decay
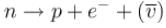
A nucleus with mass number 240 breaks into two fragments each of mass number 120, the binding energy per nucleon of unfragmented nuclei is 7.6 MeV while that of fragments is 8.5 MeV. The total gain in the Binding Energy in the process is :
Which of the following is true regarding the beam energies required to probe the inside of nucleus


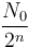
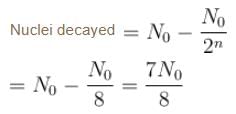
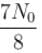
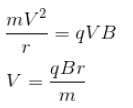
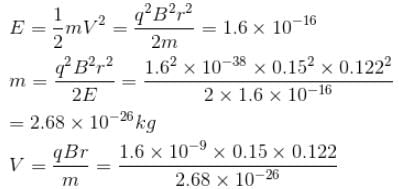

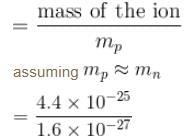
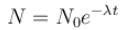
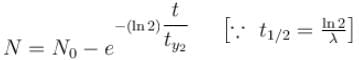
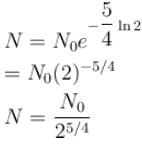
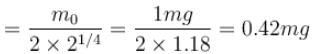

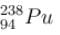
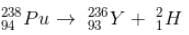
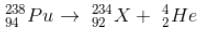
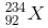 has a lower mass number & from the B.E/nucleon v/s A plot, it can be concluded that
has a lower mass number & from the B.E/nucleon v/s A plot, it can be concluded that 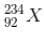 will have a greater B.E./nucleon as compared to
will have a greater B.E./nucleon as compared to