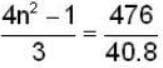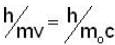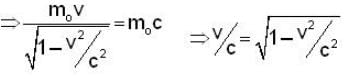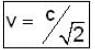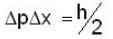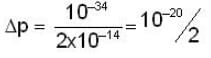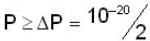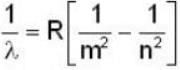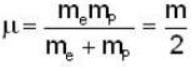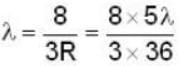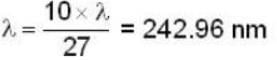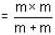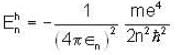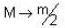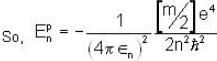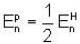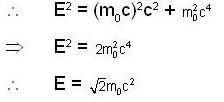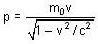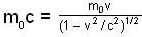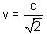IIT JAM Physics Mock Test - 8 - Physics MCQ
30 Questions MCQ Test IIT JAM Physics Mock Test Series 2025 - IIT JAM Physics Mock Test - 8
How much energy must be given to an election to accelerate it to 0.95c?
The de-Broglie wavelength of a proton and an α - paiticle are equal. The ratio of their velocities is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Let the potential energy of a hydrogen atom in the ground state be zero. Then its energy in the first excited state will be
A clock keeps correct time, with what speed should it be moved to an observer so that it may appear to loose 5 minutes in 24 hours?
The rate of radiation of a black body at 00C is E Watt. Then the rate of radiation of this black body at 2730C will be :
A harmonic oscillator has the wave function 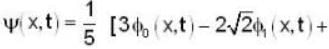
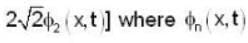 is the eigenfunction belonging to the nth energy eigenvalue
is the eigenfunction belonging to the nth energy eigenvalue 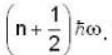 The expectation value <E> of energy for the state ψ(x,t) is
The expectation value <E> of energy for the state ψ(x,t) is
Consider a quantum mechanical system with three linear operators 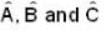 which are related by
which are related by 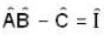 Where
Where 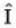 is the unit operator. If
is the unit operator. If  the
the 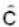 must be
must be
Electric field component of an electro magnetic radiation varies with time as 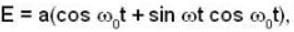 where a is a constant and the values of the ω and
where a is a constant and the values of the ω and  respectively. This radiation falls on a metal of work function 2 eV. The maximum kinetic Energy of photoelectrons is.
respectively. This radiation falls on a metal of work function 2 eV. The maximum kinetic Energy of photoelectrons is.
The electrons x and y are moving with velocities 0.1 C and 0.8C respectively. The ratio of energies ΔEX / ΔEy required to increase the speed of each by 0.1 C is given approximately by
A particle A of mass mA decays into two particles, one of which is B with mass mB and the other massless. The magnitude of the momentum of particle B using relativistic kinematics in the first-frame of the particle A is
A driver is being fined for overlooking a red signal. The driver claimed that instead of red colour (v0 = 4.8 * 1014 Hz) he saw green (v = 5.6 * 1014 Hz) because of Dopper effect. The judge fined the driver for speed at the rate of Rs. 1/- for each km/h he exceeded the speed limit of 80 km/h. What is the fine?
In a Devisson - Germer experiment a collimated beam of electrons of energy 54eV at a normal incidence on a given crystal, shows a peak appears at the same value of reflection angle, then the energy of the neutrons must be
The photo electric threshold of certain metal is 2750 A°. Find the maximum kinetic energy of the electrons. 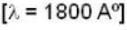
The velocity of an electron in the ground state of a hydrogen atom is VH. If Vp be the velocity of an electron in the ground state of positronium, then
A beam of atoms moving in a certain direction can be slowed down if they absorb photons from a laser beam moving in the opposite direction and subsequently spontaneously emit photons isotropically. For a beam of sodium atoms (mass number A = 23) with speed 600 m/sec. If a laser beam of wavelength 589 nm is used the number of such absorption and emission cycles needed to bring a sodium atom to rest would be approximately________
An α-particle and a proton are fired through the same magnetic field which is perpendicular to their velocity vectors. The α-particle and the proton move such that radius of curvature of their path is same. What is the ratio of their de-Broglie wavelengths?
In a collision between a photon and a free electron, which is correct, if all the photon energy were transferred to the electron.
Denote the computer of two matrices A and by [A, B] = AB - BA and anti commutator by {A,B} = AB + BA
If {A.B} = 0 we can write [A,B,C]
The activity of a radioactive sample is decreased by 25% of the initial value after 30 days. The half - life (in days) of the sample is approximately
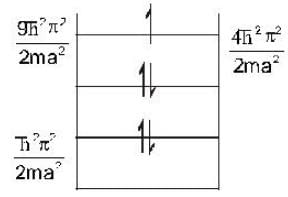
Five identical non-interacting particles, each of spin 1/2 and mass m, are moving in a one-dimensional infinite potential well given by
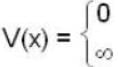
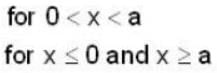
The energy of the lowest energy state is
The frequency of electron in Bohr orbit is given by f1 revolutions/sec. The frequency of electron in the n-th orbit for n > 1 is
An electron is moving inside a uniformly charged sphere with total charge +ve. The uncertainty principle ΔPxΔx ≥ n is used for finding the smallest radius rmin of the charged sphere such that the kinetic energy of the electron is equal to its potential energy on the surface of the sphere. The rmin is approximately in the range .
The Compton effect experiment photons of energy h v around material of atomic number Z. The change in wavelength can be
1.5 mW of 400 nm light is directed at a photo electric cell. If 0.1 % of the incident photon produce e-s. all the photo e- s reach the opposite plate, so the current in the photo cell is equal to______ .
In a hydrogen - like atom, an election is the ground state requires 476 ev to reach an excited level with quantum number 2n. If it makes a transition from this level to a lower level with quantum number n, it emits a photon of energy 40.8 eV. Find the atomic number Z of the element
The speed of an electron, whose de Broglie wavelength is equal to its Compton wavelength, is (C is the speed of light)
A neutron of mass mn = 10-27 kg is moving inside a nucleus. Assume the nucleus to be a cubical box of size 10-14 m with impenetrable walls. Take 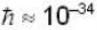 Js and 1 MeV ≈ 10-13 J . An estimate of the energy in Mev of the neutron is
Js and 1 MeV ≈ 10-13 J . An estimate of the energy in Mev of the neutron is
The longest wavelength in the Balmer series of hydrogen atom is given as 656 nm positronium can be considered as a system of an electron and a positron in which both these particles orbit each other. What will be the longest wavelength in the lyman series for positronium?
Positronium is a hydrogen like bound state of a position and electron. If the nth energy level of hydrogen atoms is given by 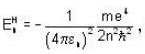 the nth energy
the nth energy 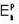 of the positronium will be equal to
of the positronium will be equal to
A particle has rest mass m0 and momentum m0c, where c is the velocity of light. The total energy and the velocity of the particle are respectively.
|
4 docs|21 tests
|


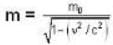

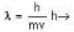 Planck's constant
Planck's constant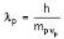
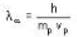
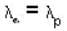
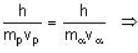
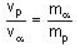
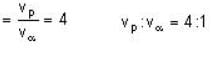
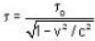
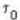 is proper time = 1440 minutes in a day and t = 1445 minutes measured by a stationary obseiver.
is proper time = 1440 minutes in a day and t = 1445 minutes measured by a stationary obseiver.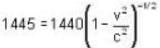
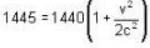
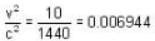
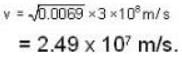
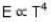
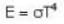 ⇒
⇒ 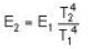
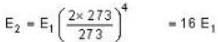
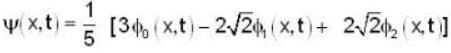 for Harmonic oscillator energy eigen value is
for Harmonic oscillator energy eigen value is 
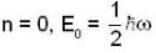
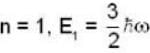
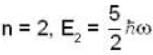
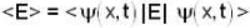
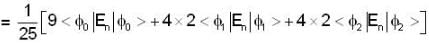
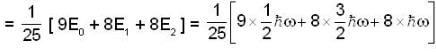
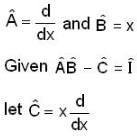
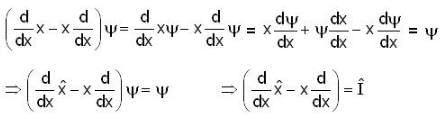
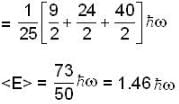
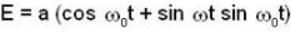
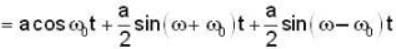
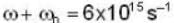
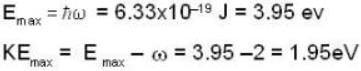
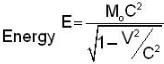
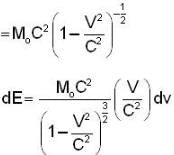
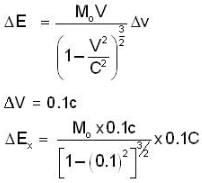
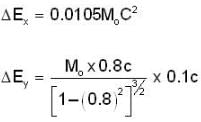

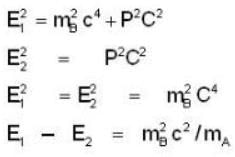
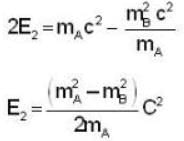
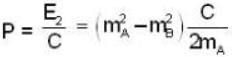
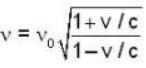
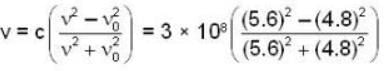 = 4.59 107 m /sec. = 1.65 x108 km/h
= 4.59 107 m /sec. = 1.65 x108 km/h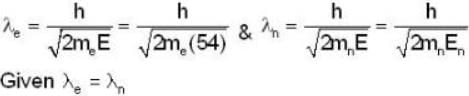
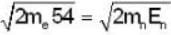
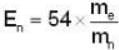

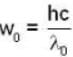
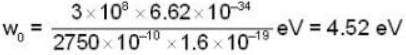
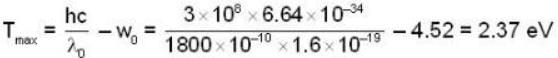
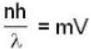
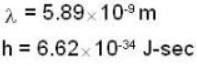
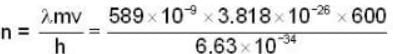
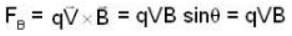
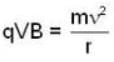 ⇒ mv = qBr
⇒ mv = qBr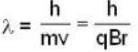


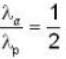
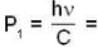 momentum of photon
momentum of photon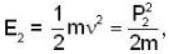 energy of electron
energy of electron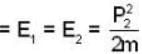
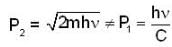
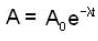
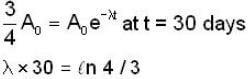
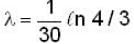
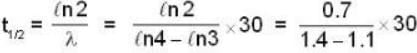
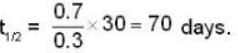
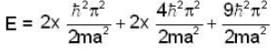
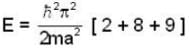

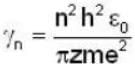
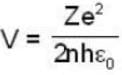
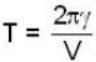
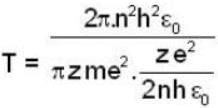
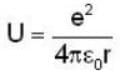
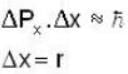
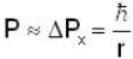
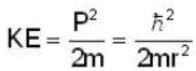
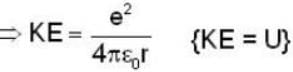
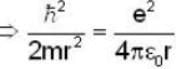
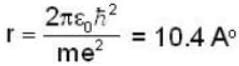
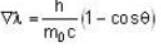
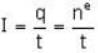 ...(i)
...(i)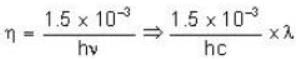
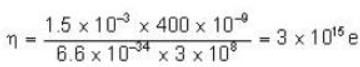
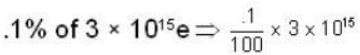
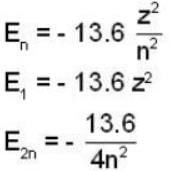
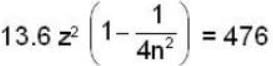 ...(i)
...(i)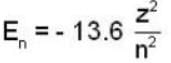
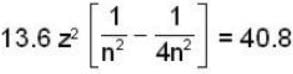
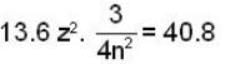 ...(ii)
...(ii)