Test: Entropy - 2 - Mechanical Engineering MCQ
10 Questions MCQ Test Thermodynamics - Test: Entropy - 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In statistical thermodynamics, entropy is defined as
1. measure of irreversibility of a system
2. a universal property
3. degree of randomness
4. thermodynamic probability of disorderness
Which of the above is/are correct
1. measure of irreversibility of a system
2. a universal property
3. degree of randomness
4. thermodynamic probability of disorderness
Energy tends over to gravitate to a lower degree of intensity so long as it undergoes no transformation.
Which law of thermodynamics is related to this statement
Consider the following statements:
In an irreversible process
1. entropy always increases
2. the sum of the entropy of all bodies taking part in a process always increases
3. once created, entropy can not be destroyed
Which among the above are correct?
One kg of water at room temperature is brought into contact with a high temperature thermal reservoir. The entropy change of the universe is
For a thermodynamics cycle to be irreversible, it is necessary that
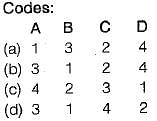
Match List-I with List-ll and select the correct answer using the codes given below
List-I
A. Mechanical work
B. 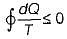
C. Zeroth law
D. H - TS
List-II
1. Clausius-Clapeyron equation
2. Gibb’s equation
3. High grade energy
4. Concept of temperature
|
29 videos|65 docs|36 tests
|
|
29 videos|65 docs|36 tests
|


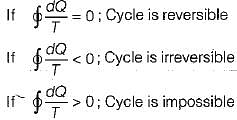
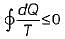
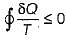 is known as Clausius inequality
is known as Clausius inequality