Mechanical Engineering Exam > Mechanical Engineering Tests > Topicwise Question Bank for Mechanical Engineering > Test: Properties of Gases - 2 - Mechanical Engineering MCQ
Test: Properties of Gases - 2 - Mechanical Engineering MCQ
Test Description
10 Questions MCQ Test Topicwise Question Bank for Mechanical Engineering - Test: Properties of Gases - 2
Test: Properties of Gases - 2 for Mechanical Engineering 2024 is part of Topicwise Question Bank for Mechanical Engineering preparation. The Test: Properties of Gases - 2 questions and answers have been
prepared according to the Mechanical Engineering exam syllabus.The Test: Properties of Gases - 2 MCQs are made for Mechanical Engineering 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Properties of Gases - 2 below.
Solutions of Test: Properties of Gases - 2 questions in English are available as part of our Topicwise Question Bank for Mechanical Engineering for Mechanical Engineering & Test: Properties of Gases - 2 solutions in
Hindi for Topicwise Question Bank for Mechanical Engineering course. Download more important topics, notes, lectures and mock
test series for Mechanical Engineering Exam by signing up for free. Attempt Test: Properties of Gases - 2 | 10 questions in 30 minutes | Mock test for Mechanical Engineering preparation | Free important questions MCQ to study Topicwise Question Bank for Mechanical Engineering for Mechanical Engineering Exam | Download free PDF with solutions
Detailed Solution for Test: Properties of Gases - 2 - Question 1
Test: Properties of Gases - 2 - Question 2
Which of the following conditions are favourable for liquefaction of a gas?
Detailed Solution for Test: Properties of Gases - 2 - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Properties of Gases - 2 - Question 3
If value of n is zero in a polytropic process PVn = C, then the process is known as constant
Detailed Solution for Test: Properties of Gases - 2 - Question 3
Test: Properties of Gases - 2 - Question 4
For an ideal gas the value of Joule Thomson coefficient is
Detailed Solution for Test: Properties of Gases - 2 - Question 4
Test: Properties of Gases - 2 - Question 5
Change in internal energy of small temperature change ΔT for ideal gas is expressed by the relation (for unit mass)
Test: Properties of Gases - 2 - Question 6
Change in enthalpy for small temperature change ΔT for ideal gas for unit mass is expressed by the relation
Detailed Solution for Test: Properties of Gases - 2 - Question 8
Test: Properties of Gases - 2 - Question 9
The relation pvγ= constant, where γ is the ratio of the specific heats of ideal gas is applicable to
Test: Properties of Gases - 2 - Question 10
When a gas is to be stored, the type of compression that would be ideal is
Detailed Solution for Test: Properties of Gases - 2 - Question 10
|
45 videos|314 tests
|
Information about Test: Properties of Gases - 2 Page
In this test you can find the Exam questions for Test: Properties of Gases - 2 solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Properties of Gases - 2, EduRev gives you an ample number of Online tests for practice
|
45 videos|314 tests
|
Download as PDF


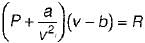 is known as
is known as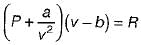 is know n as Van der Waal’s equation
is know n as Van der Waal’s equation 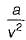 = Forces of cohesion
= Forces of cohesion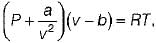 The unit of b is
The unit of b is