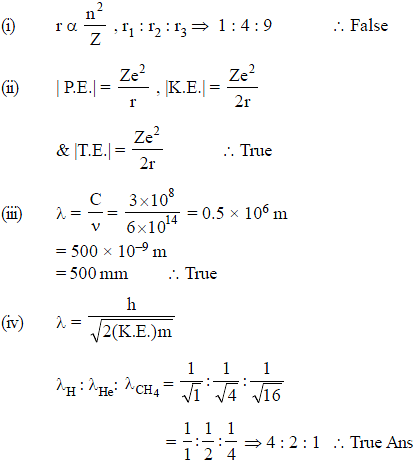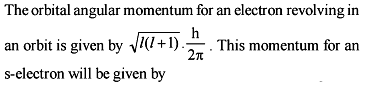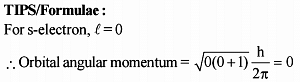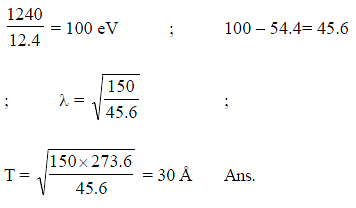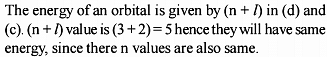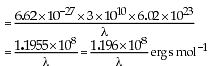Atomic Structure- 2 - JEE MCQ
30 Questions MCQ Test Chemistry for JEE Main & Advanced - Atomic Structure- 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
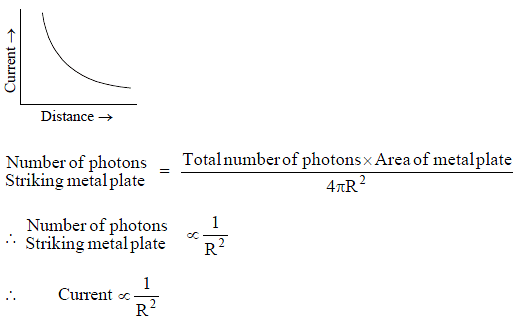
Which of the following curve correctly represent the photocurrent as a function of distance between the point source and the small metal plate?
Give the correct order of initials T (true) or F (false) for following statements.
(i) The ratio of the radii of the first three Bohr orbit of hydrogen atom is 1 : 8 : 27
(ii) The ratio of magnitude of Total Energy : Kinetic Energy : Potential Energy for any orbit is 1 : 1 : 2
(iii) The frequency of a green light is 6 × 1014 Hz, then its wavelength is 500 nm
(iv) The ratio of de-broglie wavelength of a 'H' atom, 'He' atom and CH4 molecule moving with equal kinetic energy is 4 : 2 : 1
A photon of wavelength 12.4 nm is used to emit an electron from the ground state of He+. Calculate debroglie wavelength (in Angstrom) of emitted electron.
(Given: hc = 1240 nm.eV)
[Fill your answer by multiplying it with (273.6)1/2]
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|