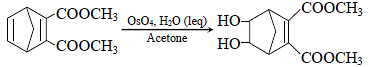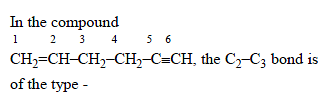Hydrocarbon - 1 - JEE MCQ
30 Questions MCQ Test Chemistry for JEE Main & Advanced - Hydrocarbon - 1
During dehydration of alcohols to alkenes by heating with conc. H2SO4 the initiation step is-
[AIEEE-2003]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
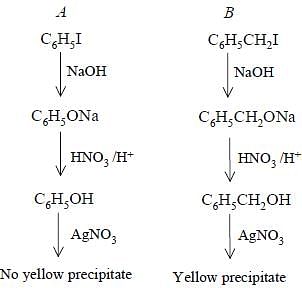
Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.
Which one of the following statements is true for this experiment ?
[AIEEE-2003]
Which one of the following statements is true for this experiment ?

The alkene formed as a major product in the above elimination reaction is
[AIEEE 2006]
The no. of isomeric sodium salt that will be required to obtain neopentane.
Which sodium salt will be heated with sodalime to obtain propane -
During the preparation of ethane by Kolbe's electrolytic method using inert electrode the pH of the electrolyte -
In the above reaction if we take methylene chloride and isopropylidene chloride then products are -
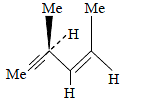
Hydrogenation of the above compound in the presence of poisoned pallodium catalyst gives
What would be the product when ethene is oxidised with cold dil. KMnO4 solution -
Which one of the following alkenes will react fastest with H2 under catalytic hydrogenation condition -
The reduction of 4-octyne with H2 in the presence of Pd/CaCO3 – quinoline gives (as a major product) -
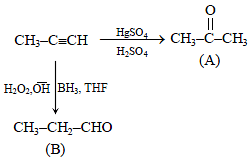
When propyne is treated with aqueous H2SO4 in presence of HgSO4, the major product is -
BrCH2–CH2–CH2Br reacts with Na in the presence of ether at 100ºC to produce -
How much volume of air will be needed for complete combustion of 10 lit. of ethane -
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|