O.P Tandon Test: Hydrocarbons - JEE MCQ
25 Questions MCQ Test Chemistry for JEE Main & Advanced - O.P Tandon Test: Hydrocarbons
in the Wurtz reaction method of preparation of alkanes, the stoichiometric coefficient of Sodium is
When two hydrogen atoms in benzene are replaced by two similar or different monovalent atoms or groups, how many different position isomers are possible?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following reactions of methane is incomplete combustion?
The hydrocarbon which can react with sodium in liquid ammonia is
The number of possible conformational isomers of ethane is
Among the following compounds the one that is most reactive towards electrophilic nitration is
Aromatics are charachterized by the presence of _____ π electrons in the ring where n is an integer (n = 0, 1, 2, . . .).
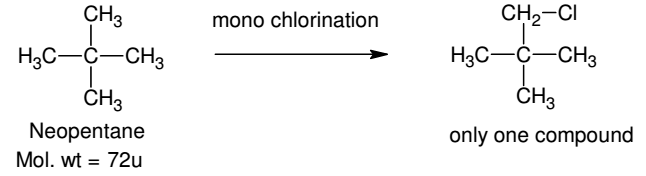
Which branched chain isomer of the hydrocarbon with molecular mass 72u gives only one isomer of mono substituted alkyl halide?
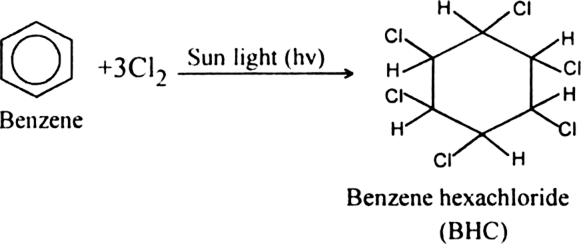
Benzene reacts with chlorine in sunlight to give a final product
What is the product obtained on electrolyzing potassium fumarate?
What is the commercially used method to produce benzene?
What product is obtained by heating ethylidene chloride with alcoholic KOH?
in Friedel-Crafts alkylation reaction, when benzene is treated with an alkyl halide in the presence of a catalyst, alkylbenene is formed. The catalyst used is
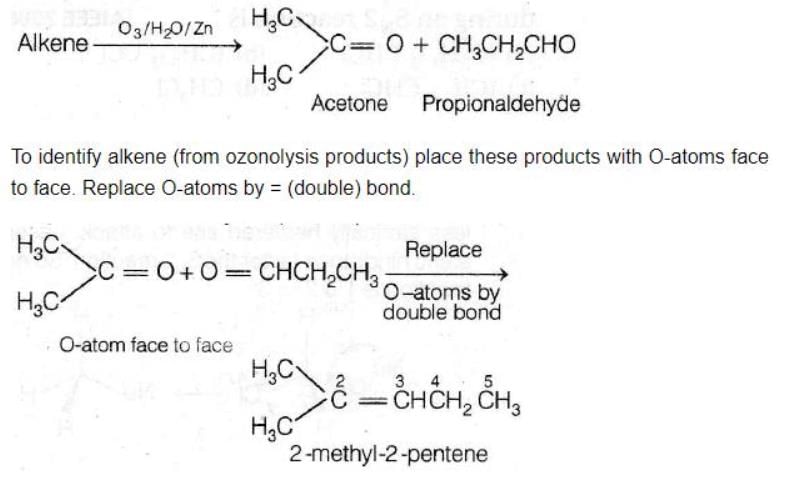
Ozonolysis of an organic compound 'A' produces acetone and propionaldehyde in equimolar mixture. Identify 'A' from the following compounds
[AIEEE 2011]
Cyclohexene on ozonolysis followed by reaction with zinc dust and water gives compound E.Compound E on further treatment with aqueous KOH yields compound F.Compound F is
What product is obtained by passing ethanol vapours over heated alumina?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|