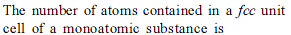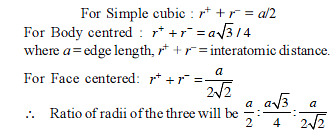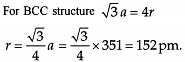Solid State - 2 - JEE MCQ
30 Questions MCQ Test Chemistry for JEE Main & Advanced - Solid State - 2
If Z is the number of atoms in the unit cell that represents the closest packing sequence ABC ABC … , the number of tetrahedral voids in the unit cell is equal to -
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
An element X (At. wt. = 80 g/mol) having fcc structure, calculate no. of unit cells in 8 gm of X :
The radius of the Na+ is 95 pm and that of Cl– ion is 181 pm. Predict the co-ordination number of Na+
If the distance between Na+ and Cl–1 ions in NaCl crystal is 265 pm, then edge length of the unit cell will be ?
In a normal spinel types structure, the oxide ions are arranged in ccp whereas 1/8 tetrahedral holes are occupied by Zn2+ ions and 50% of octahedral holes are occupied by Fe3+ ions. The formula of the compound is –
In an FCC crystal, which of the following shaded planes contains the following type of arrangement of atoms ?

Element ‘B’ forms ccp structure and ‘A’ occupies half of the octahedral voids, while oxygen atoms occupy all the tetrahedral voids. The structure of bimetallic oxide is -
TlAl(SO4)2 . xH2O is bcc with 'a'= 1.22 nm. If the density of the solid is 2.32 g/cc, then the value of x is (Given : NA = 6 × 1023; at. wt. : Tl = 204, Al = 27, S = 32, O = 16, H = 1)
A group IV A element with a density of 11.35 g/cm3 crystallise in a face centered cubic lattice whose unit cell edge length is 4.95Å. Calculate its atomic mass -
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|