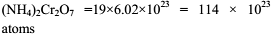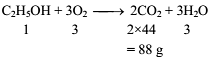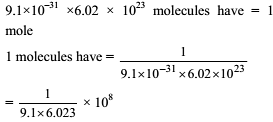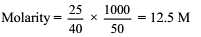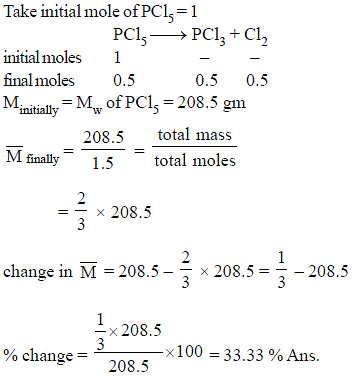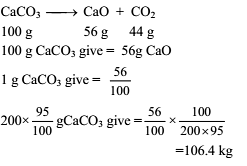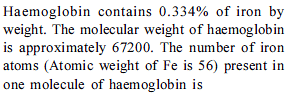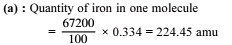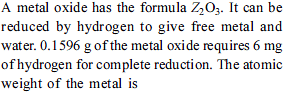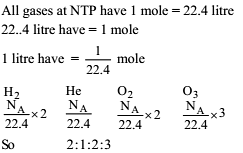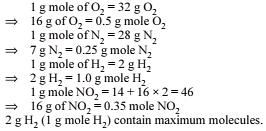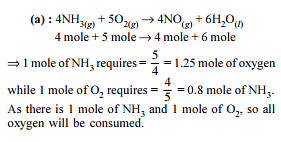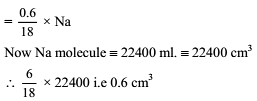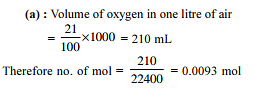Some Basic Concepts of Chemistry - 1 - JEE MCQ
30 Questions MCQ Test Chemistry for JEE Main & Advanced - Some Basic Concepts of Chemistry - 1
Total number of atoms of all elements present in 1 mole of ammonium dichromate is ?
If one mole of ethanol (C2H5OH) completely burns to carbon dioxide and water, the weight of carbon dioxide formed is about -
(C2H5OH + 3O2 → 2CO2 + 3H2O)
(C2H5OH + 3O2 → 2CO2 + 3H2O)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The iron atoms in 1720 amu of ferric ferrocyanide is .....................
The hydrated salt Na2SO4.nH2O, undergoes 55% loss in weight on heating and becomes anhydrous. The value of n will be
Insulin contains 3.4% sulphur. What will be the minimum molecular weight of insulin ?
How many moles of electron weight one kilogram ?
The largest number of molecules is present in
25 g of NaOH is dissolved in 50 mL of water. The molarity of the solution is
Calculate percentage change in Mavg of the mixture, if PCl5 undergo 50% decomposition.
PCl5 → PCl3 + Cl2
Calculate the weight of lime (CaO) obtained by heating 200 kg of 95% pure lime stone (CaCO3).
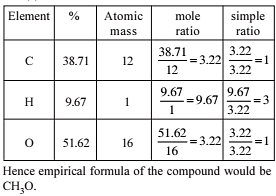
A carbon compound containing carbon and oxygen has molar mass equal to 288. On analysis it is found to contain 50% by mass of
each element. Therefore molecular formula of the compound is -
Four one litre flasks are separately filled with the gases hydrogen, helium, oxygen and ozone at the same room temperature and pressure.
The ratio of total number of atoms of these gases present in the different flasks would be
What volume of 0.4-M FeCl3.6H2O will contain 600 mg of Fe3+ ?
At 100ºC and 1 atm, if the density of liquid water is 1.0 g cm–3 and that of water vapor is 0.0006 g cm–3, then the volume occupied by water molecules in 1 litre of steam at that temperature is -
Volume of a gas at NTP is 1.12 × 10–7 c.c. The number of molecules in it will be -
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|