JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Adsorption, Adsorption isotherms - JEE MCQ
Test: Adsorption, Adsorption isotherms - JEE MCQ
Test Description
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Adsorption, Adsorption isotherms
Test: Adsorption, Adsorption isotherms for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Adsorption, Adsorption isotherms questions and answers have been
prepared according to the JEE exam syllabus.The Test: Adsorption, Adsorption isotherms MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Adsorption, Adsorption isotherms below.
Solutions of Test: Adsorption, Adsorption isotherms questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Adsorption, Adsorption isotherms solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Adsorption, Adsorption isotherms | 20 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Test: Adsorption, Adsorption isotherms - Question 1
The volume of gases 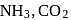 and
and 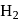 adsorbed by one gram of charcoal at
adsorbed by one gram of charcoal at  are in order of:
are in order of:
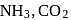 and
and 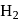 adsorbed by one gram of charcoal at
adsorbed by one gram of charcoal at  are in order of:
are in order of:
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 1
Test: Adsorption, Adsorption isotherms - Question 2
The heat evolved in chemisorption lies in the range (in kJ/mol) of:
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Adsorption, Adsorption isotherms - Question 3
 gram of oxygen is adsorbed on
gram of oxygen is adsorbed on  of metal powder. What volume of oxygen adsorbed per gram of the adsorbent at
of metal powder. What volume of oxygen adsorbed per gram of the adsorbent at  and
and 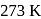 ?
?
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 3
Test: Adsorption, Adsorption isotherms - Question 4
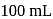 of
of 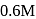 acetic acid is shaken with
acetic acid is shaken with  activated carbon. The final concentration of the solution after adsorption is
activated carbon. The final concentration of the solution after adsorption is 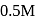 . What is the amount of acetic acid adsorbed per gram of carbon?
. What is the amount of acetic acid adsorbed per gram of carbon?
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 4
Test: Adsorption, Adsorption isotherms - Question 5
Although, nitrogen does not adsorb on a surface at room temperature, it adsorbs on the surface at  . Which one of the following statements is correct?
. Which one of the following statements is correct?
 . Which one of the following statements is correct?
. Which one of the following statements is correct?
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 5
Test: Adsorption, Adsorption isotherms - Question 6
Which of the following statements is not correct?
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 6
Test: Adsorption, Adsorption isotherms - Question 7
Which one of the following statements is correct for adsorption of solutes on solids in solutions?
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 7
Test: Adsorption, Adsorption isotherms - Question 8
Methylene blue, from its aqueous solution, is adsorbed on activated charcoal at  . For this process, which of the following statement is correct?
. For this process, which of the following statement is correct?
 . For this process, which of the following statement is correct?
. For this process, which of the following statement is correct?
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 8
Test: Adsorption, Adsorption isotherms - Question 9
In an experiment, 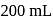 of
of  oxalic acid is shaken with
oxalic acid is shaken with 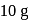 of activated charcoal and filtered. The concentration of the filtrate is reduced to
of activated charcoal and filtered. The concentration of the filtrate is reduced to 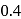
 . The amount of adsorption
. The amount of adsorption 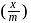 is
is
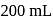 of
of  oxalic acid is shaken with
oxalic acid is shaken with 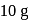 of activated charcoal and filtered. The concentration of the filtrate is reduced to
of activated charcoal and filtered. The concentration of the filtrate is reduced to 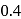
 . The amount of adsorption
. The amount of adsorption 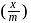 is
is
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 9
Test: Adsorption, Adsorption isotherms - Question 10
Which of the following statements regarding difference between adsorption and absorption is incorrect?
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 10
Test: Adsorption, Adsorption isotherms - Question 11
Which gas will be adsorbed on a solid to greater extent?
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 11
Test: Adsorption, Adsorption isotherms - Question 12
Which of the following statements is correct for the spontaneous adsorption of a gas?
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 12
Test: Adsorption, Adsorption isotherms - Question 13
For adsorption, the thermodynamic requirement is that
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 13
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 14
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 15
Test: Adsorption, Adsorption isotherms - Question 16
For the graph below, select correct order of temperature?


Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 16
Test: Adsorption, Adsorption isotherms - Question 17
Which one of the following equations represents Freundlich adsorption isotherm?
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 17
Test: Adsorption, Adsorption isotherms - Question 18
At the high pressure, Langmuir adsorption isotherm takes the form
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 18
Test: Adsorption, Adsorption isotherms - Question 19
According to Langmuir adsorption isotherm, the amount of gas adsorbed at very high pressures
Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 19
Test: Adsorption, Adsorption isotherms - Question 20
The plot of  (y-axis) and
(y-axis) and  -axis) is a straight line inclined at an angle of
-axis) is a straight line inclined at an angle of  . When the intercept,
. When the intercept, 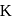 is 10 and pressure is
is 10 and pressure is  the amount of solute in grams adsorbed per gram of adsorbent
the amount of solute in grams adsorbed per gram of adsorbent 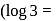

 (y-axis) and
(y-axis) and  -axis) is a straight line inclined at an angle of
-axis) is a straight line inclined at an angle of  . When the intercept,
. When the intercept, 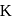 is 10 and pressure is
is 10 and pressure is  the amount of solute in grams adsorbed per gram of adsorbent
the amount of solute in grams adsorbed per gram of adsorbent 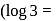

Detailed Solution for Test: Adsorption, Adsorption isotherms - Question 20
|
352 videos|596 docs|309 tests
|
Information about Test: Adsorption, Adsorption isotherms Page
In this test you can find the Exam questions for Test: Adsorption, Adsorption isotherms solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Adsorption, Adsorption isotherms, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF


 to
to 
 per gram of adsorbent
per gram of adsorbent 
 per gram of
per gram of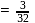
 per gram of adsorbent
per gram of adsorbent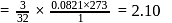
 charcoal
charcoal 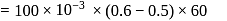

 physical absorption takes place effectively so multimolecular layers formed.
physical absorption takes place effectively so multimolecular layers formed. surface area of adsorbate.
surface area of adsorbate.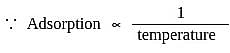

 amount of adsorbent
amount of adsorbent 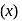 , adsorbed on given mass
, adsorbed on given mass  of adsorbate and
of adsorbate and  an integer. Thus, plot of
an integer. Thus, plot of  gives a straight line upward slope.
gives a straight line upward slope.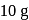 charcoal
charcoal

 and
and 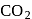 , which liquefy more easily are adsorbed more readily than the permanent gases like
, which liquefy more easily are adsorbed more readily than the permanent gases like 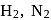 and
and  which do not liquefy easily.
which do not liquefy easily. and for a spontaneous change
and for a spontaneous change  hence
hence  should be highly negative which is clear from the equation
should be highly negative which is clear from the equation 

 is highly negative
is highly negative  will also be
will also be 
 Extent of adsorption
Extent of adsorption 

























