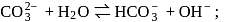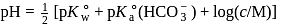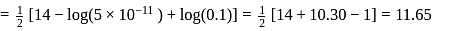Test: Buffer solutions, Salt Hydrolysis - JEE MCQ
10 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Buffer solutions, Salt Hydrolysis
Fixed volume of 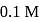 benzoic acid solution is added into
benzoic acid solution is added into 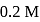 sodium benzoate solution and formed a
sodium benzoate solution and formed a 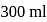 , resultant acidic buffer solution. If
, resultant acidic buffer solution. If 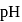 of this buffer solution is
of this buffer solution is 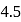 then find added volume of benzoic acid –
then find added volume of benzoic acid –
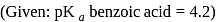
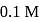 benzoic acid solution is added into
benzoic acid solution is added into 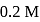 sodium benzoate solution and formed a
sodium benzoate solution and formed a 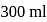 , resultant acidic buffer solution. If
, resultant acidic buffer solution. If 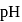 of this buffer solution is
of this buffer solution is 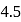 then find added volume of benzoic acid –
then find added volume of benzoic acid –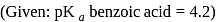
Assuming that the buffer in the blood 
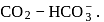 Calculate the ratio of conjugate base to acid necessary to maintain blood at its proper
Calculate the ratio of conjugate base to acid necessary to maintain blood at its proper 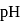 of
of 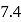
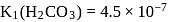
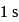
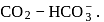 Calculate the ratio of conjugate base to acid necessary to maintain blood at its proper
Calculate the ratio of conjugate base to acid necessary to maintain blood at its proper 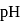 of
of 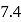
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In a buffer solution having equal concentrations of 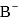 and HB the value of
and HB the value of 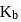 for
for 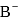 is
is 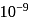 . The pH of buffer solution is
. The pH of buffer solution is
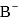 and HB the value of
and HB the value of 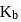 for
for 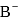 is
is 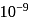 . The pH of buffer solution is
. The pH of buffer solution isFor preparing a buffer solution of  by mixing sodium acetate and acetic acid, the ratio of the concentration of salt and acid should be
by mixing sodium acetate and acetic acid, the ratio of the concentration of salt and acid should be 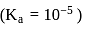
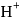 ion concentration in a solution prepared by mixing
ion concentration in a solution prepared by mixing 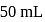 of
of 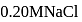 ,
, 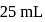 of
of 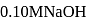 and
and 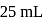 of
of 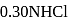 ?
?
The dissociation constant of a weak acid is 1 × 10−4. In order to prepare a buffer solution with a pH = 5 the [Salt] /[Acid] ratio should be
Calculate the 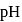 of
of 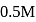 aqueous solution of
aqueous solution of 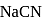 , the
, the 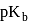 of
of 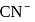 is
is 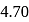
What is the percentage hydrolysis of 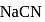 in
in 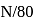 solution when the dissociation constant for
solution when the dissociation constant for 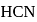 is
is 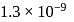 and
and 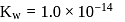
Given: Kb (dimethylamine) = 7.4 × 10−4M. A buffer is made by mixing (CH3)2NH and (CH3)2NH2Cl. The range of buffer to which this buffer can be used is
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|


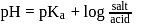
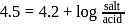
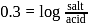
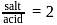
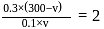
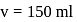
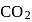 with
with 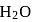 forms
forms 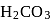
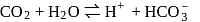
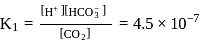

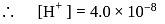
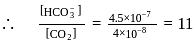
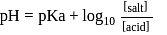
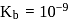
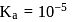
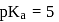
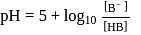
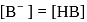
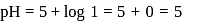
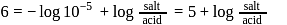
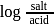 must be 1.
must be 1. 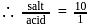 or
or 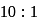
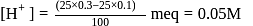
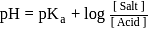
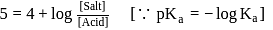
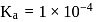
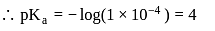
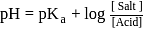
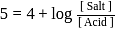
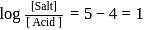
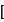 Salt
Salt  Acid
Acid 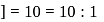
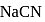 is a salt of strong base and weak acid ;
is a salt of strong base and weak acid ; 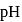
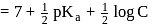
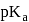 for
for 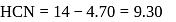
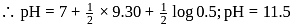
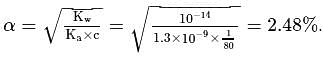 .
.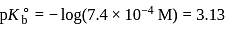
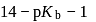 to
to 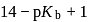 , i.e.
, i.e. 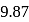 to
to 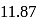
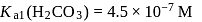 and
and 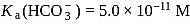 , the
, the 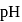 of
of 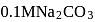 solution at
solution at 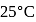 will be
will be