JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Carbohydrates - JEE MCQ
Test: Carbohydrates - JEE MCQ
Test Description
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Carbohydrates
Test: Carbohydrates for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Carbohydrates questions and answers have been
prepared according to the JEE exam syllabus.The Test: Carbohydrates MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Carbohydrates below.
Solutions of Test: Carbohydrates questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Carbohydrates solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Carbohydrates | 20 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Detailed Solution for Test: Carbohydrates - Question 1
Test: Carbohydrates - Question 2
Which of the following compounds is a recipient of the one carbon fragments that tetra hydro folate receives and transfers?
Detailed Solution for Test: Carbohydrates - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Detailed Solution for Test: Carbohydrates - Question 3
Detailed Solution for Test: Carbohydrates - Question 4
Test: Carbohydrates - Question 5
An organic compound with the formula  forms a yellow crystalline solid with phenylhydrazine and gives a mixture of sorbitol and mannitol when reduced with sodium. Which among the following could be the compound?
forms a yellow crystalline solid with phenylhydrazine and gives a mixture of sorbitol and mannitol when reduced with sodium. Which among the following could be the compound?
 forms a yellow crystalline solid with phenylhydrazine and gives a mixture of sorbitol and mannitol when reduced with sodium. Which among the following could be the compound?
forms a yellow crystalline solid with phenylhydrazine and gives a mixture of sorbitol and mannitol when reduced with sodium. Which among the following could be the compound?
Detailed Solution for Test: Carbohydrates - Question 5
Test: Carbohydrates - Question 6
When  -D-glucose and
-D-glucose and  -D-glucose are dissolved in water in two separate beakers I and II respectively and allowed to stand, then -
-D-glucose are dissolved in water in two separate beakers I and II respectively and allowed to stand, then -
 -D-glucose and
-D-glucose and  -D-glucose are dissolved in water in two separate beakers I and II respectively and allowed to stand, then -
-D-glucose are dissolved in water in two separate beakers I and II respectively and allowed to stand, then -
Detailed Solution for Test: Carbohydrates - Question 6
Test: Carbohydrates - Question 7
Which of the following pairs can be distinguished by Fehling's solution ?
Detailed Solution for Test: Carbohydrates - Question 7
Test: Carbohydrates - Question 8
Identify the correct statements from the following:
 When
When  is hydrolysed, adenine and thymine are obtained in equal quantities.
is hydrolysed, adenine and thymine are obtained in equal quantities.
 When
When  is hydrolysed, adenine and uracil are obtained in equal quantities.
is hydrolysed, adenine and uracil are obtained in equal quantities.
 Amylose is branched polymer with
Amylose is branched polymer with  and
and  glycosidic linkages.
glycosidic linkages.
 Addison disease is due to the abnormal functioning of adrenal cortex.
Addison disease is due to the abnormal functioning of adrenal cortex.
 When
When  is hydrolysed, adenine and thymine are obtained in equal quantities.
is hydrolysed, adenine and thymine are obtained in equal quantities. When
When  is hydrolysed, adenine and uracil are obtained in equal quantities.
is hydrolysed, adenine and uracil are obtained in equal quantities. Amylose is branched polymer with
Amylose is branched polymer with  and
and  glycosidic linkages.
glycosidic linkages. Addison disease is due to the abnormal functioning of adrenal cortex.
Addison disease is due to the abnormal functioning of adrenal cortex.
Detailed Solution for Test: Carbohydrates - Question 8
Test: Carbohydrates - Question 9
Observe the following statements.
I. Sucrose has glycosidic linkage.
II. Cellulose is present in both plants and animals.
III. Lactose contains D-galactose and D-glucose units.
Which of the following statements are correct?
I. Sucrose has glycosidic linkage.
II. Cellulose is present in both plants and animals.
III. Lactose contains D-galactose and D-glucose units.
Which of the following statements are correct?
Detailed Solution for Test: Carbohydrates - Question 9
Test: Carbohydrates - Question 10
Which of the following carbohydrates is not related to  -glucose?
-glucose?
 -glucose?
-glucose?
Detailed Solution for Test: Carbohydrates - Question 10
Test: Carbohydrates - Question 11
Glucose-D has a great tendency to be converted into cyclic isomer. Which two carbon atoms get joined through '  ' to form this hemiacetal ?
' to form this hemiacetal ?
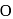 ' to form this hemiacetal ?
' to form this hemiacetal ?
Detailed Solution for Test: Carbohydrates - Question 11
Detailed Solution for Test: Carbohydrates - Question 12
Detailed Solution for Test: Carbohydrates - Question 13
Test: Carbohydrates - Question 14
Which of the following evolves carbon dioxide, on oxidation with periodate?
Detailed Solution for Test: Carbohydrates - Question 14
Test: Carbohydrates - Question 15
Which of the following carbohydrates has a glycosidic linkage?
Detailed Solution for Test: Carbohydrates - Question 15
Detailed Solution for Test: Carbohydrates - Question 16
Test: Carbohydrates - Question 17
A certain compound gives negative test with ninhydrin and positive test with Benedict's solution. The compound is
Detailed Solution for Test: Carbohydrates - Question 17
Test: Carbohydrates - Question 18
Glucose forms many derivatives. The derivative which will help to prove the furanose structure is
Detailed Solution for Test: Carbohydrates - Question 18
Detailed Solution for Test: Carbohydrates - Question 19
Detailed Solution for Test: Carbohydrates - Question 20
|
352 videos|596 docs|309 tests
|
Information about Test: Carbohydrates Page
In this test you can find the Exam questions for Test: Carbohydrates solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Carbohydrates, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF



 from in which the OH at C1 in aldose and C2 in ketoses lies towards the right and
from in which the OH at C1 in aldose and C2 in ketoses lies towards the right and  form in which it lies towards left. Thus glucose,fructose,ribose,etc.,all exist
form in which it lies towards left. Thus glucose,fructose,ribose,etc.,all exist 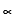 and
and  form. Glucose exists in two forms
form. Glucose exists in two forms 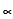 -D glucose and
-D glucose and  -D glucose
-D glucose 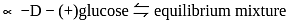
 As a result of cyclization the anomeric (C-1) becomes asymmetric and the newly formed - OH group may be either on left or on a right in Fischer projections thus resulting in the formation of two isomers (anomers). The isomers having - OH group to the left of the C-1 is designsted
As a result of cyclization the anomeric (C-1) becomes asymmetric and the newly formed - OH group may be either on left or on a right in Fischer projections thus resulting in the formation of two isomers (anomers). The isomers having - OH group to the left of the C-1 is designsted  and other having - OH group on the right as
and other having - OH group on the right as 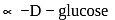 .
.
 - glucose is
- glucose is
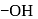 group at (carbon) C-2, we give D and L configuration. For right side
group at (carbon) C-2, we give D and L configuration. For right side  -glucose For left side
-glucose For left side  L-(-)-glucose
L-(-)-glucose -D-glucose or
-D-glucose or 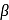 -D-glucose when dissolved in water and allowed to stand, following equilibrium is established.
-D-glucose when dissolved in water and allowed to stand, following equilibrium is established.  -D-glucose
-D-glucose  Open chain form
Open chain form  -D-glucose
-D-glucose











 -form falls, while that of
-form falls, while that of  -form increases until a constant value of
-form increases until a constant value of  is reached at equilibrium. This phenomenon is known as mutarotation.
is reached at equilibrium. This phenomenon is known as mutarotation. groups (one due to glucose and another due to fructose) are not free, so it can't attain -CHO group. Hence it will not respond Fehling's solution.
groups (one due to glucose and another due to fructose) are not free, so it can't attain -CHO group. Hence it will not respond Fehling's solution. When
When  is hydrolysed, adenine and thymine are obtained in equal quantities.
is hydrolysed, adenine and thymine are obtained in equal quantities. When
When  is hydrolysed, adenine and uracil are obtained in equal quantities.
is hydrolysed, adenine and uracil are obtained in equal quantities.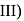 Amylose is branched polymer with
Amylose is branched polymer with 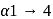 and
and  glycosidic linkages.
glycosidic linkages. Addison disease is due to the abnormal functioning of adrenal cortex.
Addison disease is due to the abnormal functioning of adrenal cortex. .
. and
and  .
. -glucose is
-glucose is In the
In the  family the more laevorotatory anomer is named
family the more laevorotatory anomer is named 

 epimers, it implies that these are epimers and diastereomers too.
epimers, it implies that these are epimers and diastereomers too. group on both sides is removed as
group on both sides is removed as  during oxidation with periodate.
during oxidation with periodate. glucose residues are joined by a glycosidic linkage between the
glucose residues are joined by a glycosidic linkage between the  anomeric form of
anomeric form of  on one sugar and the hydroxyl oxygen atom on
on one sugar and the hydroxyl oxygen atom on  of the adjacent sugar. Such a linkage is called an
of the adjacent sugar. Such a linkage is called an  glycosidic bond.
glycosidic bond. glucose is
glucose is
 -
- 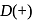 -glucose.A chiral carbon has four different groups attached. -D-Glucose has no plane of symmetry, so all the carbon atoms are different. There are five chiral carbons.
-glucose.A chiral carbon has four different groups attached. -D-Glucose has no plane of symmetry, so all the carbon atoms are different. There are five chiral carbons. +Citrate ions)
+Citrate ions) and
and  carbon. When both of them react with three molecules of phenylhydrazine, the formation of osazone crystals makes the difference between the carbon atoms in their chain and hence, in such way it helps to prove the fructose structure.
carbon. When both of them react with three molecules of phenylhydrazine, the formation of osazone crystals makes the difference between the carbon atoms in their chain and hence, in such way it helps to prove the fructose structure.
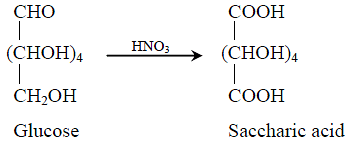
 . It is derived by oxidizing a sugar such as glucose with nitric acid.
. It is derived by oxidizing a sugar such as glucose with nitric acid.