JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Collision Theory, Energy of Activation - JEE MCQ
Test: Collision Theory, Energy of Activation - JEE MCQ
Test Description
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Collision Theory, Energy of Activation
Test: Collision Theory, Energy of Activation for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Collision Theory, Energy of Activation questions and answers have been
prepared according to the JEE exam syllabus.The Test: Collision Theory, Energy of Activation MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Collision Theory, Energy of Activation below.
Solutions of Test: Collision Theory, Energy of Activation questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Collision Theory, Energy of Activation solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Collision Theory, Energy of Activation | 20 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Test: Collision Theory, Energy of Activation - Question 1
A first order reaction is half-completed in 45 minutes. How long does it need for 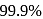 of the reaction to be completed?
of the reaction to be completed?
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 1
Test: Collision Theory, Energy of Activation - Question 2
At a certain temperature, the first order rate constant k1 is found to be smaller than the second order rate constant k2. If the energy of activation E1 of the first order reaction is greater than energy of activation E2 of the second order reaction, then with increase in temperature.
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Collision Theory, Energy of Activation - Question 3
A reaction which is of first order w.r.t. reactant  , has a rate constant
, has a rate constant  . If we start with
. If we start with 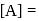
 , when would [A] reach the value of
, when would [A] reach the value of 
 , has a rate constant
, has a rate constant  . If we start with
. If we start with 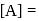
 , when would [A] reach the value of
, when would [A] reach the value of 
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 3
Test: Collision Theory, Energy of Activation - Question 4
The rate of a first order reaction is 
 at
at  concentration of the reactant. The halflife of the reaction is
concentration of the reactant. The halflife of the reaction is

 at
at  concentration of the reactant. The halflife of the reaction is
concentration of the reactant. The halflife of the reaction is
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 4
Test: Collision Theory, Energy of Activation - Question 5
For a first order reaction  the reaction rate at reactant concentration of
the reaction rate at reactant concentration of 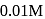 is found to be
is found to be  . The half life period of the reaction is
. The half life period of the reaction is
 the reaction rate at reactant concentration of
the reaction rate at reactant concentration of 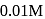 is found to be
is found to be  . The half life period of the reaction is
. The half life period of the reaction is
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 5
Test: Collision Theory, Energy of Activation - Question 6
A reaction proceeds by first order,  of this reaction was completed in 32 min. The time required for
of this reaction was completed in 32 min. The time required for  completion is
completion is
 of this reaction was completed in 32 min. The time required for
of this reaction was completed in 32 min. The time required for  completion is
completion is
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 6
Test: Collision Theory, Energy of Activation - Question 7
In the reaction  , rate constant is
, rate constant is 
 . If we start with
. If we start with 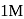 of A then conc. of
of A then conc. of  and B after 10 minuter are respectively.
and B after 10 minuter are respectively.
 , rate constant is
, rate constant is 
 . If we start with
. If we start with 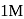 of A then conc. of
of A then conc. of  and B after 10 minuter are respectively.
and B after 10 minuter are respectively.
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 7
Test: Collision Theory, Energy of Activation - Question 8
The half life for the virus inactivation if in the beginning 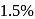 of the virus is inactivated per minute is (Given: The reaction is of first order)
of the virus is inactivated per minute is (Given: The reaction is of first order)
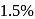 of the virus is inactivated per minute is (Given: The reaction is of first order)
of the virus is inactivated per minute is (Given: The reaction is of first order)
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 8
Test: Collision Theory, Energy of Activation - Question 9
At 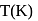 , if the rate constant of a first order reaction is
, if the rate constant of a first order reaction is  , the time to reduce the initial concentration of the reactant to
, the time to reduce the initial concentration of the reactant to  in seconds is :
in seconds is :
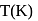 , if the rate constant of a first order reaction is
, if the rate constant of a first order reaction is  , the time to reduce the initial concentration of the reactant to
, the time to reduce the initial concentration of the reactant to  in seconds is :
in seconds is :
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 9
Test: Collision Theory, Energy of Activation - Question 10
The rate constant of a reaction with a virus is  . Time required for the virus to become
. Time required for the virus to become  inactivated is
inactivated is
 . Time required for the virus to become
. Time required for the virus to become  inactivated is
inactivated is
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 10
Test: Collision Theory, Energy of Activation - Question 11
A Geigger Muller counter is used to study the radioactive process. In the absence of radioactive substance 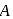 , it counts 3 disintegration per second (dps). At the start in the presence of
, it counts 3 disintegration per second (dps). At the start in the presence of  , it records 23 dps; and after 10 min 13 dps,
, it records 23 dps; and after 10 min 13 dps,
(i) What does it count after
(ii) What is the halflife of A?
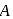 , it counts 3 disintegration per second (dps). At the start in the presence of
, it counts 3 disintegration per second (dps). At the start in the presence of  , it records 23 dps; and after 10 min 13 dps,
, it records 23 dps; and after 10 min 13 dps,(i) What does it count after

(ii) What is the halflife of A?
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 11
Test: Collision Theory, Energy of Activation - Question 12
The rate equation for a reaction,

is Rate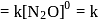 . If the initial concentration of the reactant is
. If the initial concentration of the reactant is 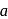 mol
mol  , the half-life period of the reaction is
, the half-life period of the reaction is

is Rate
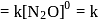 . If the initial concentration of the reactant is
. If the initial concentration of the reactant is 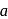 mol
mol  , the half-life period of the reaction is
, the half-life period of the reaction is
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 12
Test: Collision Theory, Energy of Activation - Question 13
Half-lives of first-order and zeroth order reactions are same. Ratio of rates at the start of nreaction is
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 13
Test: Collision Theory, Energy of Activation - Question 14
The plot of concentration of the reactant Vs time for a reaction is a straight line with a negative slope. The reaction follows a rate equation of
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 14
Test: Collision Theory, Energy of Activation - Question 15
The reaction  is started with
is started with  of L. After 30 and 90 minutes
of L. After 30 and 90 minutes 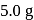 and
and  of
of 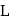 respectively are left. The order of the reaction is
respectively are left. The order of the reaction is
 is started with
is started with  of L. After 30 and 90 minutes
of L. After 30 and 90 minutes 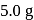 and
and  of
of 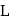 respectively are left. The order of the reaction is
respectively are left. The order of the reaction is
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 15
Test: Collision Theory, Energy of Activation - Question 16
The plot that represents the zero order reaction is:
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 16
Test: Collision Theory, Energy of Activation - Question 17
For a first order reaction, a plot of log(a−x) against time is a straight line with a negative slope equal to
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 17
Test: Collision Theory, Energy of Activation - Question 18
In the presence of an acid, the initial concentration of cane sugar was reduced from  to
to  in 5 hours and from
in 5 hours and from 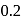 to
to  in 10 hours. The reaction is of :
in 10 hours. The reaction is of :
 to
to  in 5 hours and from
in 5 hours and from 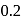 to
to  in 10 hours. The reaction is of :
in 10 hours. The reaction is of :
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 18
Test: Collision Theory, Energy of Activation - Question 19
For the decomposition of  at
at  , the following data were obtained
, the following data were obtained

The order of reaction is :
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 19
Test: Collision Theory, Energy of Activation - Question 20
The hypothetical reaction  follows the following mechanism
follows the following mechanism 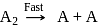 ,
,  The order of the overall reaction is
The order of the overall reaction is
Detailed Solution for Test: Collision Theory, Energy of Activation - Question 20
|
352 videos|596 docs|309 tests
|
Information about Test: Collision Theory, Energy of Activation Page
In this test you can find the Exam questions for Test: Collision Theory, Energy of Activation solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Collision Theory, Energy of Activation, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF


 further
further
 hours.
hours.

 Products
Products or
or 


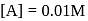
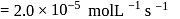



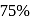 reaction gets completed in 32
reaction gets completed in 32 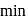



 to get the value of time required for
to get the value of time required for  completion of reaction
completion of reaction







 th, we use the formula:
th, we use the formula:
 , we have:
, we have:
 , we get approximately 500 seconds.
, we get approximately 500 seconds.
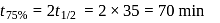
 dps is zero error, hence
dps is zero error, hence 
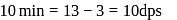

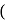 recorded
recorded 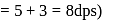


 zeroth
zeroth 
 first
first 




 therefore
therefore  is 30 minutes. In 90 minutes the amount is reduced to
is 30 minutes. In 90 minutes the amount is reduced to  i.e.
i.e.  Here
Here  True for 1st order reaction.
True for 1st order reaction.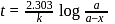





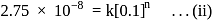
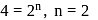
 ;
; (Fast);
(Fast); (Slow)
(Slow) put value of
put value of  from Ist reaction since
from Ist reaction since  is intermediate
is intermediate
 Rate law equation
Rate law equation 
 Order
Order 















