Test: Concentration of the Ore - JEE MCQ
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Concentration of the Ore
Which of the following statements regarding metallurgy of iron is incorrect?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following condition favours the reduction of a metal oxide to metal?
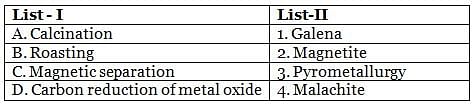
Match the items of List-I with those of List-II and choose the correct option given below.
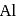 by electrolysis?
by electrolysis?
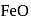 in blast furnace , it is converted to
in blast furnace , it is converted to 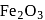 by roasting so that
by roasting so that
Identify the correct statements from the following:
i. In the extraction of 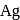 and
and 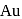 , zinc is used as reducing agent.
, zinc is used as reducing agent.
ii. Impure zinc can be refined by distillation method.
iii. Malachite is an ore of nickel.
When a metal is to be extracted from its ore and the gangue associated with the ore is silica, then
1. Reduction of alumina to give aluminium by magnesium is thermodynamically feasible.
2. The point of intersection of
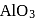 and
and 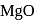 curves in Ellingham diagram is below
curves in Ellingham diagram is below 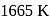 .
.3. Use of magnesium as reducing agent in metallurgy of aluminium is economical.
4. Ellingham diagram represents the graphical plot of Gibbs energy vs temperature for the formation of the oxides of common metals and reducing agents.
Which of the following examples is not correctly matched?
One of the processes used for concentration of ores is Froth floatation process. This process is generally used for concentration of sulphide ores. Sometimes in this process we add 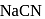 as a depressant.
as a depressant. 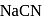 is generally added in case of
is generally added in case of 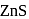 and PbS minerals. What is the purpose of addition of NaCN during the process of Froth floatation ?
and PbS minerals. What is the purpose of addition of NaCN during the process of Froth floatation ?
(i) Froth-floatation is used for removing gangue from sulphide ore.
(ii) Cresols are used to stabilise the froth.
(iii) Sodium cyanide can be used as depressant for preferential separation.
(iv) Aniline can be used as froth enhancer.
Which of the following reactions is an example for calcination process?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|


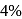 carbon and impurities like
carbon and impurities like 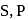 Mn etc., in small amount.
Mn etc., in small amount.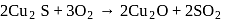
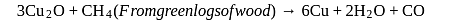
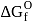 of most of the sulphides are greater than those of
of most of the sulphides are greater than those of 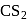 and
and 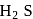 , therefore neither
, therefore neither 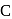 nor
nor 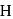 can reduce metal sulphide to metal. Further, the standard free energies of formation of oxide are much less than those of
can reduce metal sulphide to metal. Further, the standard free energies of formation of oxide are much less than those of 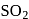 . Hence oxidation of metal sulphides to metal oxide is thermodynamically favourable.
. Hence oxidation of metal sulphides to metal oxide is thermodynamically favourable.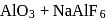 as elecurolyse, carbon
as elecurolyse, carbon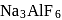 or
or 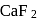 (cryolite or fluorspar), which lowers the melting point of alumina and brings conductivity.
(cryolite or fluorspar), which lowers the melting point of alumina and brings conductivity.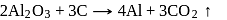
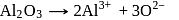
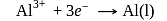 At anode
At anode 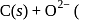 melt
melt 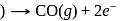
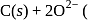 melt
melt 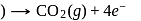
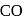 and
and 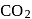 . Hence, option (a) is the correct answer.
. Hence, option (a) is the correct answer.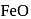 is capable forming slag with
is capable forming slag with 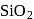
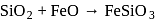
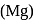 as reducing agent in metallurgy of aluminium
as reducing agent in metallurgy of aluminium 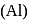 is uneconomical. So, statement 3 is not correct.
is uneconomical. So, statement 3 is not correct.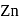 and
and 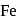 occur only in combined state, as these metals are available in nature mostly in the form of sulphide and oxides respectively.
occur only in combined state, as these metals are available in nature mostly in the form of sulphide and oxides respectively.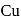 and
and 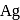 occur in both native and combined state.
occur in both native and combined state.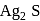 is an ore of silver. Silver is extracted from argentite by the mac-Arthur and Forest process (leaching process).
is an ore of silver. Silver is extracted from argentite by the mac-Arthur and Forest process (leaching process).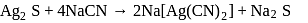
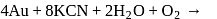
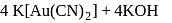
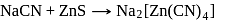
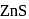 and due to this
and due to this 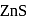 is prevented from the froth formation while PbS form froth.
is prevented from the froth formation while PbS form froth.














