Test: Conjugation and Aromaticity - JEE MCQ
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Conjugation and Aromaticity
Pyrrole and pyridine both are basic and form salts with acids?

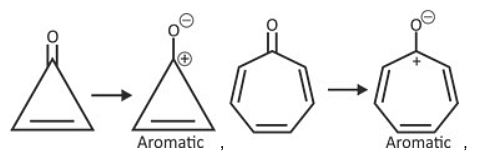
Which of the following statement is true regarding the aromatic character of the four species?

Which of the following statement is true regarding the aromatic character of the four species?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The correct stability order of the following resonance structures is


Correct stability order of resonating structure is:
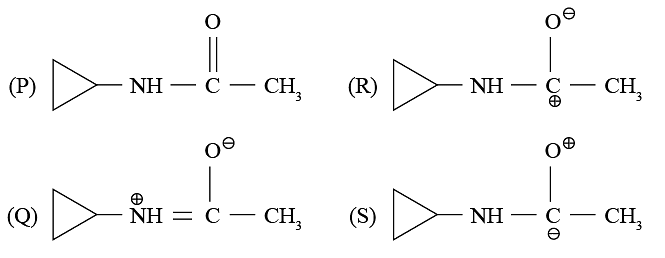
Pick up the correct statement  regarding the following resonating structures of the anilinium ion
regarding the following resonating structures of the anilinium ion

Which of the following resonance structure is lowest in energy?

All the hydrocarbons shown are very weak acids. One, however, is far more acidic than the others. Which one is the strongest acid?
The correct order of acid strength of the following compounds
A. Phenol
B. p-Cresol
C. m-Nitrophenol
D. p - Nitrophenol is
Arrange the following compounds in order of decreasing acidity:

The increasing order of acidity of the following carboxylic acids is

The maximum π-electron-density is present between which of the numbered carbon atoms?

Find out total number of compounds which are more stable in its ionic form
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|