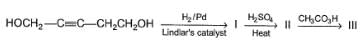Test: Epoxides - JEE MCQ
22 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Epoxides
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
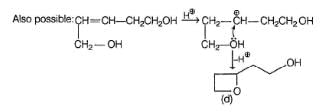
The most appropriate reagents that can bring about the following transformation is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
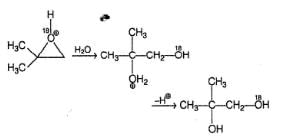
In the reaction given below,
The correct deduction concerning X and Y is
An alkene on treatment with meta chloro perbenzoic acid undergoes epoxidation reaction to give oxirane. What is the correct order of increasing reactivity of the following in the above mentioned epoxidation reaction?
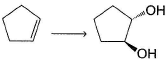
Which of the following compound gives more than one oxirane upon epoxidation with Ag2O/heat?
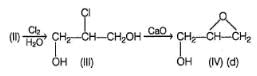
The reaction given beiow that gives oxirane as the major organic product is
One or More than One Options Correct Type
Direction (Q, Nos. 9-14)This section contains 6 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
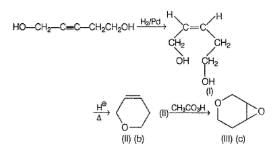
Consider the following reaction sequence,
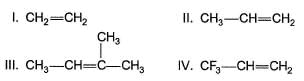
Q.
The intermediate products (I - IV) in the above reaction is/are
The reagent that can bring about the following transformation in good yield is/are
In the reaction below,
The correct statement concerning the above reaction is/are
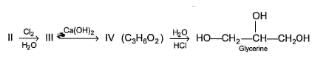
Consider the following reaction sequence,
The correct statem ent regarding X, Y and Z is/are
Comprehension Type
Direction (Q. Nos. 15-17) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Passage
Oxirane is a reactive ether, undergo nucleophilic attack at a-carbon in the presence of both acidic and basic medium. The generalised mechanism in the two medium are :
I. Acidic medium
II. Basic medium
As shown above, in acidic medium, nucleophilic attack occur at more substituted α-carbon while in basic medium, nucleophilic attack occur preferably at less substituted α-carbon.
Q.
In the reaction given below, th e final major organic product Y is
Oxirane is a reactive ether, undergo nucleophilic attack at a-carbon in the presence of both acidic and basic medium. The generalised mechanism in the two medium are :
I. Acidic medium
II. Basic medium
As shown above, in acidic medium, nucleophilic attack occur at more substituted α-carbon while in basic medium, nucleophilic attack occur preferably at less substituted α-carbon.
Q.
If X is heated with concentrated H2SO4 a cyclic compound is formed which is
Oxirane is a reactive ether, undergo nucleophilic attack at a-carbon in the presence of both acidic and basic medium. The generalised mechanism in the two medium are :
I. Acidic medium
II. Basic medium
As shown above, in acidic medium, nucleophilic attack occur at more substituted α-carbon while in basic medium, nucleophilic attack occur preferably at less substituted α-carbon.
Q.
What would be formed if 2-phenyloxirane is treated with CH3MgBr followed by acid hydrolysis?
One Integer Value Correct Type
Direction (Q. Nos. 18-22) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
How many different ether isomers are possible for C3H6O ?
Consider the reaction given below,
Q.
How many different triols isomers are formed?
If propene undergoes oxidation to methyl oxirane, what is the net change in oxidation number of carbon atoms?
In the following reaction,
Q.
How many different stereoisomers of product formed ?
How many different arene oxide would be formed on mono epoxidation of phenanthrene?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|