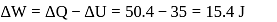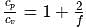Test: First law, Thermodynamic Equilibrium - JEE MCQ
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: First law, Thermodynamic Equilibrium
A gas expands adiabatically at constant pressure such that  . The value of
. The value of  of the gas will be :
of the gas will be :
 . The value of
. The value of  of the gas will be :
of the gas will be :What is the value of change in internal energy at 1 atm in the process?

Given : 
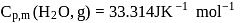
 at
at 

Given :

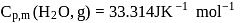
 at
at 
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
An ideal gas expands against a constant external pressure of 2.0 atmosphere from 20 litre to 40 litre and absorbs 10 kJ of heat from surrounding. What is the change in internal energy of the system? (given : 1 atm-litre = 101.3 J)
The pressure-volume diagram shows six curved paths that can be followed by the gas (connected by vertical paths). Which two of them should be part of a closed cycle if the net work done by the gas is to be its maximum positive value?

On P − V coordinates, the slope of an isothermal curve of a gas at a pressure P = 1MPa and volume V = 0.0025 m3 is equal to −400MPa/m3. If Cp/Cv = 1.4, the slope of the adiabatic curve passing through this point is :
For an ideal gas four processes are marked as 1 2,3 and 4 on  diagram as shown in figure. The amount of heat supplied to the gas in the process
diagram as shown in figure. The amount of heat supplied to the gas in the process  and 4 are
and 4 are  and
and  respectively, then correct order of heat supplied to the gas is
respectively, then correct order of heat supplied to the gas is  is process-
is process-  is process-
is process-  is adiabatic process-3 and
is adiabatic process-3 and  is process-4]
is process-4]
A system changes from the state  to
to 
 ) as shown in the figure. What is the work done by the system?
) as shown in the figure. What is the work done by the system?

If 2.5 moles of an ideal gas at a certain temperature are allowed to expand isothermally and reversibly from an initial volume of  to
to  , the work done by the gas is −16.5 kJ.
, the work done by the gas is −16.5 kJ.
The temperature (in K) of the gas is (Round off to the nearest value)

An ideal gas is subjected to cyclic process involving four thermodynamic states, the amounts of heat (Q) and work (W) involved in each of these states 

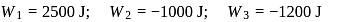

The ratio of the net work done by the gas to the total heat absorbed by the gas is 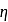 . The values of
. The values of  and
and  respectively are
respectively are
For a monoatomic gas, the value of the ratio of  and
and  is
is
If  is the change in enthalpy and
is the change in enthalpy and  the change in internal energy accompanying a gaseous reaction, then
the change in internal energy accompanying a gaseous reaction, then
An ideal gas expands adiabatically against vaccum. Which of the following is correct for the given process?
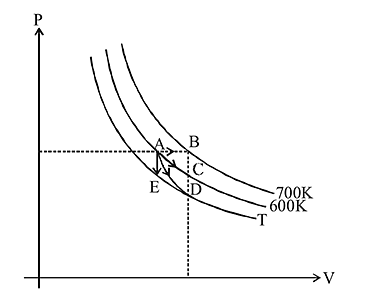
When the state of a gas adiabatically changed from an equilibrium state A to another equilibrium state B an amount of work done on the stystem is  . If the gas is taken from state
. If the gas is taken from state  to
to 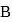 via process in which the net heat absorbed by the system is
via process in which the net heat absorbed by the system is 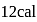 , then the net work done by the system is
, then the net work done by the system is 
The ratio γ = Cp, m/CV, m for a diatomic gaseous molecules is
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|


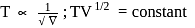
 constant
constant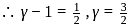










 Joule
Joule






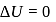 Now,
Now, 

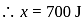







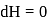


 law of thermodynamics,
law of thermodynamics,