JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Group 15 Elements: Nitrogen Group - JEE MCQ
Test: Group 15 Elements: Nitrogen Group - JEE MCQ
Test Description
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Group 15 Elements: Nitrogen Group
Test: Group 15 Elements: Nitrogen Group for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Group 15 Elements: Nitrogen Group questions and answers have been
prepared according to the JEE exam syllabus.The Test: Group 15 Elements: Nitrogen Group MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Group 15 Elements: Nitrogen Group below.
Solutions of Test: Group 15 Elements: Nitrogen Group questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Group 15 Elements: Nitrogen Group solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Group 15 Elements: Nitrogen Group | 20 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 1
Test: Group 15 Elements: Nitrogen Group - Question 2
In nitrogen family, the H-M-Hbond angle in the hydrides gradually becomes closer to 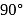 on going from
on going from 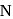 to
to  . This shows that gradually
. This shows that gradually
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 3
Test: Group 15 Elements: Nitrogen Group - Question 4
A deep brown gas is formed by mixing two colourless gases which are
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 4
Test: Group 15 Elements: Nitrogen Group - Question 5
Which of the following compounds does not exist?
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 5
Test: Group 15 Elements: Nitrogen Group - Question 6
The brown ring test for  and
and 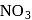 is due to the formation of complex ion with a formula
is due to the formation of complex ion with a formula
 and
and 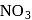 is due to the formation of complex ion with a formula
is due to the formation of complex ion with a formula
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 6
Test: Group 15 Elements: Nitrogen Group - Question 7
An element  forms compounds of the formula
forms compounds of the formula  and
and  but does not form
but does not form  .
.
Which of the following is the element 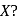
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 7
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 8
Test: Group 15 Elements: Nitrogen Group - Question 9
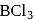 is a planar molecule whereas
is a planar molecule whereas  is pyramidal because
is pyramidal because
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 9
Test: Group 15 Elements: Nitrogen Group - Question 10
A metal  on heating in nitrogen gas gives
on heating in nitrogen gas gives  on treatment with
on treatment with  gives a colourless gas which when passed through
gives a colourless gas which when passed through  solution gives a blue colour.
solution gives a blue colour.  is
is
 on heating in nitrogen gas gives
on heating in nitrogen gas gives  on treatment with
on treatment with  gives a colourless gas which when passed through
gives a colourless gas which when passed through  solution gives a blue colour.
solution gives a blue colour.  is
is
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 10
Test: Group 15 Elements: Nitrogen Group - Question 11
Which reaction(s) among the following produce dinitrogen?


Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 11
Test: Group 15 Elements: Nitrogen Group - Question 12
Consider the following sequence of reaction.

Identify  gas :
gas :
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 12
Test: Group 15 Elements: Nitrogen Group - Question 13
Among the following compounds, which on heating do not produce  ?
?
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 13
Test: Group 15 Elements: Nitrogen Group - Question 14
Which of the following statements is not true?
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 14
Test: Group 15 Elements: Nitrogen Group - Question 15
The compounds formed by the reaction of ammonia with chlorine and iodine are respectively
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 15
Test: Group 15 Elements: Nitrogen Group - Question 16
 on heating liberates a gas. The same gas will be obtained by
on heating liberates a gas. The same gas will be obtained by
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 16
Test: Group 15 Elements: Nitrogen Group - Question 17
Among the following elements which one can not form pentahalides.
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 17
Test: Group 15 Elements: Nitrogen Group - Question 18
When sugar is treated with conc. sulphuric acid, the sugar is charred. In this process, sugar is
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 18
Test: Group 15 Elements: Nitrogen Group - Question 19
Which of the following nitrogen oxide is a neutral oxide?
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 19
Test: Group 15 Elements: Nitrogen Group - Question 20
(I) 
(II)
The incorrect statement regarding above reactions is

(II)

The incorrect statement regarding above reactions is
Detailed Solution for Test: Group 15 Elements: Nitrogen Group - Question 20
|
352 videos|596 docs|309 tests
|
Information about Test: Group 15 Elements: Nitrogen Group Page
In this test you can find the Exam questions for Test: Group 15 Elements: Nitrogen Group solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Group 15 Elements: Nitrogen Group, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF


 dil.
dil. 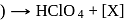
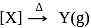
 and
and  are respectively
are respectively 

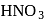 can be removed by
can be removed by which is brown
which is brown
 brown
brown ion is formed
ion is formed and not
and not 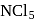 since it has no
since it has no  atomic orbitals in valence shell
atomic orbitals in valence shell there is
there is 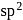 hybridisation (planar) whereas in
hybridisation (planar) whereas in  hybridisation is
hybridisation is  hence shape is pyramidal
hence shape is pyramidal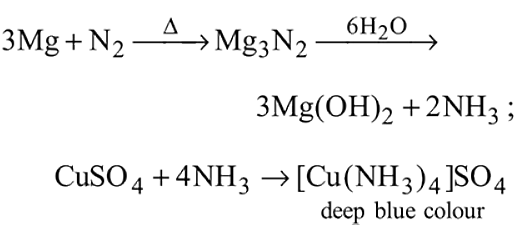
 on heating gives
on heating gives  and
and  . This reaction is a source of pure nitrogen.
. This reaction is a source of pure nitrogen.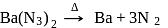

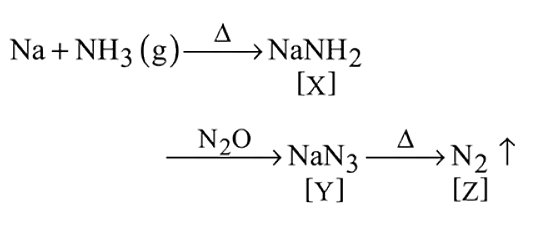

 is paramagnetic and readily dimerises to
is paramagnetic and readily dimerises to  which is diamagnetic.
which is diamagnetic. and
and  respectively as follows
respectively as follows

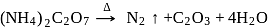
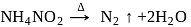

 and
and  are acidic oxide while
are acidic oxide while 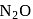 is a neutral oxide.
is a neutral oxide. , it is very polarizing and favours the formation of
, it is very polarizing and favours the formation of  .
.

















