JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Heavy Water and its Uses, Hydrogen in Industry - JEE MCQ
Test: Heavy Water and its Uses, Hydrogen in Industry - JEE MCQ
Test Description
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Heavy Water and its Uses, Hydrogen in Industry
Test: Heavy Water and its Uses, Hydrogen in Industry for JEE 2025 is part of Chemistry for JEE Main & Advanced preparation. The Test: Heavy Water and its Uses, Hydrogen in Industry questions and answers have been
prepared according to the JEE exam syllabus.The Test: Heavy Water and its Uses, Hydrogen in Industry MCQs are made for JEE 2025 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Heavy Water and its Uses, Hydrogen in Industry below.
Solutions of Test: Heavy Water and its Uses, Hydrogen in Industry questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Heavy Water and its Uses, Hydrogen in Industry solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Heavy Water and its Uses, Hydrogen in Industry | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 1
Which statement is correct for hydrogen ?
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 1
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 2
Sodium and hydrogen combine to form sodium hydride in presence of heat, what is the oxidising agent here?
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 3
The adsorption of hydrogen by metals is called
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 3
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 4
Hydrogen can be fused to form helium at
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 4
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 5
Which property among the following is same for both hydrogen and deuterium molecules?
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 5
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 6
Hydrogen molecules differs from chlorine molecule in the following respect
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 6
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 7
When two ice cubes are pressed over each other, they unite to form one cube. Which of the following forces is responsible to hold them together?
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 7
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 8
When a substance Areacts with water it produces a combustible gas  and a solution of substance C in water. When another substance D reacts with this solution of
and a solution of substance C in water. When another substance D reacts with this solution of  , it also produces the same gas
, it also produces the same gas 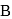 on warming but
on warming but  can produce gas
can produce gas  on reaction with dilute sulphuric acid at room temperature. A imparts a deep golden yellow colour to a smokeless flame of Bunsen burner. A,
on reaction with dilute sulphuric acid at room temperature. A imparts a deep golden yellow colour to a smokeless flame of Bunsen burner. A,  and
and  respectively are
respectively are
 and a solution of substance C in water. When another substance D reacts with this solution of
and a solution of substance C in water. When another substance D reacts with this solution of  , it also produces the same gas
, it also produces the same gas 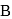 on warming but
on warming but  can produce gas
can produce gas  on reaction with dilute sulphuric acid at room temperature. A imparts a deep golden yellow colour to a smokeless flame of Bunsen burner. A,
on reaction with dilute sulphuric acid at room temperature. A imparts a deep golden yellow colour to a smokeless flame of Bunsen burner. A,  and
and  respectively are
respectively are
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 8
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 9
The hydride ion  is stronger base than its hydroxide ion
is stronger base than its hydroxide ion  . Which of the following reactions will occur if sodium hydride
. Which of the following reactions will occur if sodium hydride 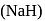 is dissolved in water?
is dissolved in water?
 is stronger base than its hydroxide ion
is stronger base than its hydroxide ion  . Which of the following reactions will occur if sodium hydride
. Which of the following reactions will occur if sodium hydride 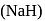 is dissolved in water?
is dissolved in water?
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 9
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 10
Hard water can block radiators due to the formation of
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 10
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 11
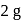 of aluminium is treated separately with excess of dilute
of aluminium is treated separately with excess of dilute  and excess of
and excess of  . The ratio of the volumes of hydrogen evolved is
. The ratio of the volumes of hydrogen evolved is
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 11
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 12
When same amount of zinc is treated separately with excess of sulphuric acid and excess of sodium hydroxide solution the ratio of volumes of hydrogen evolved is
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 12
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 13
Which of the following reactions increases production of dihydrogen from synthesis gas?
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 13
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 14
In which of the following reactions, hydrogen will not be liberated?
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 14
Test: Heavy Water and its Uses, Hydrogen in Industry - Question 15
Hydrogen gas is not obtained when zinc reacts with :
Detailed Solution for Test: Heavy Water and its Uses, Hydrogen in Industry - Question 15
|
352 videos|596 docs|309 tests
|
Information about Test: Heavy Water and its Uses, Hydrogen in Industry Page
In this test you can find the Exam questions for Test: Heavy Water and its Uses, Hydrogen in Industry solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Heavy Water and its Uses, Hydrogen in Industry, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF


 , hydrogen has
, hydrogen has  oxidation no. In
oxidation no. In  , hydrogen has
, hydrogen has  oxidation no.
oxidation no. to
to 
 , the nuclei may have sufficient energy to overcome the repulsive forces and thus fuse. This is why, fusion reactions are also called thermonuclear reactions.
, the nuclei may have sufficient energy to overcome the repulsive forces and thus fuse. This is why, fusion reactions are also called thermonuclear reactions.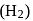 and deuterium
and deuterium 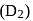 molecules have same bond length but have different bond energy, melting point and boiling point.
molecules have same bond length but have different bond energy, melting point and boiling point.



 . Since
. Since 
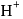 to form
to form 





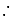 Ratio of volumes of
Ratio of volumes of 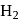 evolved is
evolved is 

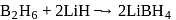
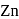 reacts with dil.
reacts with dil.  , dil.
, dil.  and hot
and hot  solution.
solution.














