Test: Hybrididation and VSEPR theory - JEE MCQ
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Hybrididation and VSEPR theory
In compounds of type ECl3, where E = B,P, As or Bi, the angles Cl − E − Cl for different E are in the order.
For which of the following sets of geometry, both axial and equatorial positions are present?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
 . The formula and shape of
. The formula and shape of  are :
are :
The bond angle between two hybrid orbitals is 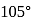 Calculate the percentage of
Calculate the percentage of  -character of hybrid orbital.
-character of hybrid orbital.
Main axis of a diatomic molecule is Z. AO's  and
and 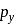 overlap to form which of the following orbitals?
overlap to form which of the following orbitals?

i. According to VSEPR theory,
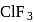 and
and 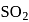 are shown
are shown  and
and  type molecules respectively.
type molecules respectively.ii.
 has "See-saw" shape.
has "See-saw" shape.iii.
 and
and  have same shape.
have same shape.The statements which are not correct are
The compound MX4 is tetrahedral. The number of ∠XMX formed in the compound are
In a regular octahedral molecule, MX6 the number of X−M−X bonds at 180∘ is
Which of the following molecular orbitals has two nodal planes?
In the reaction  , the change in hybridisation is from
, the change in hybridisation is from
Which of the following represents the given mode of hybridisation sp2 − sp2 − sp − sp from left to right ?
(i)
 being an ionic compound is a good conductor of electricity in the solid state.
being an ionic compound is a good conductor of electricity in the solid state.(ii) In canonical structures there is no difference in the arrangement of atoms.
(iii) Hybrid orbitals form stronger bonds than pure orbitals.
(iv) VSEPR theory can explain the square planar geometry of
 .
.
 and one p-orbital we get
and one p-orbital we get
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|




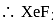 undergoes
undergoes  hybridisation, whereas
hybridisation, whereas  undergoes
undergoes  hybridisation.
hybridisation.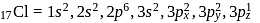


 . Its shape is pentagonal bipyramidal.
. Its shape is pentagonal bipyramidal.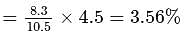
 and
and 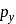 orbitals do not have proper orientation to overlap and hence no bond is formed.
orbitals do not have proper orientation to overlap and hence no bond is formed.
 has 3 bond pairs and 2 lone pairs thus it is
has 3 bond pairs and 2 lone pairs thus it is  type molecule whereas
type molecule whereas 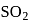 has
has  bond pairs and one lone pair so it is
bond pairs and one lone pair so it is  type molecule.
type molecule. has a linear shape because it has 2 bond pairs only whereas
has a linear shape because it has 2 bond pairs only whereas  has 2 bond pairs with one lone pair thus it has a bent shape.
has 2 bond pairs with one lone pair thus it has a bent shape.


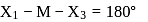


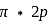



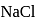 dissolves in water, the bond is broken and sodium and chlorine separate forming ions like
dissolves in water, the bond is broken and sodium and chlorine separate forming ions like  and
and  . Therefore, having free ions, it is able to conduct electricity. So, the option (1) is incorrect.
. Therefore, having free ions, it is able to conduct electricity. So, the option (1) is incorrect.
 -bonds at
-bonds at  -atom
-atom 
 -atom
-atom 

 .
. is linear
is linear
 bonds at
bonds at  -atom
-atom 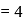


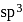 .
. is tetrahedral.
is tetrahedral. does not obey octet rule as in it S-atom has 12 electrons in its valence shell.
does not obey octet rule as in it S-atom has 12 electrons in its valence shell.
 in
in 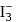 undergoes
undergoes 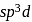 hybridisation giving a linear shape with three lone pairs at equatorial positions. Beryllium chloride has linear structure with
hybridisation giving a linear shape with three lone pairs at equatorial positions. Beryllium chloride has linear structure with  hybridisation of Be atom.
hybridisation of Be atom. forms a linear polymeric structure. There is no hydrogen bonding in
forms a linear polymeric structure. There is no hydrogen bonding in  form zigzag structure.
form zigzag structure.  has the structure in which each molecule is linked to four other molecules.
has the structure in which each molecule is linked to four other molecules. -hybrid orbitals is
-hybrid orbitals is 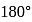 .
. is
is















