Test: Introduction to Transition Metals - JEE MCQ
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Introduction to Transition Metals
Outof [TiF6]2−, [CoF6]3−, Cu2Cl2 and [NiCl4]2− (Z of Ti = 22, Co = 27, Cu = 29, Ni = 28), the colourless species are:
The basic character of the transition metal monoxides follows the order (Atomic Nos., Ti = 22, V = 23, Cr = 24, Fe = 26)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following statement is incorrect?
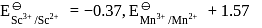

Which of the following statements is incorrect?
A metal M and its compound can give the following observable changes in a consequence of reactions

The blue complex formed on addition of conc.  solution to a
solution to a  salt solution has the structure?
salt solution has the structure?
 is added to oxalic acid, the decolourisation is slow in the beginning but becomes instantaneous after sometime because
is added to oxalic acid, the decolourisation is slow in the beginning but becomes instantaneous after sometime because
Number of electrons transfered in each case when  acts as an oxidising agent to give
acts as an oxidising agent to give  ,
, 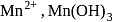 and
and  are respectively
are respectively
Chloro compound of Vanadium has only spin magnetic moment of  . This Vanadium chloride has the formula:
. This Vanadium chloride has the formula:
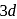 -series has highest third ionisation enthalpy?
-series has highest third ionisation enthalpy?
In which of the tollowing ions, d-d transition is not possible ?
In a reaction the ferrous (Fe+2) ion is oxidised to ferric (Fe+3) ion. The equivalent weight of the ion in the above reaction is equal to
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|


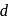 -orbitals which permits the
-orbitals which permits the  excitation of electrons.
excitation of electrons. is in
is in  O.S.
O.S.  -colourless
-colourless is in
is in  coloured
coloured is in
is in  O.S.
O.S.  colourless
colourless is in
is in 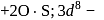 coloured
coloured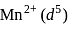 is more stable than
is more stable than  , thus
, thus 

 is hygroscopic.
is hygroscopic.
 acts as autocatalyst.
acts as autocatalyst.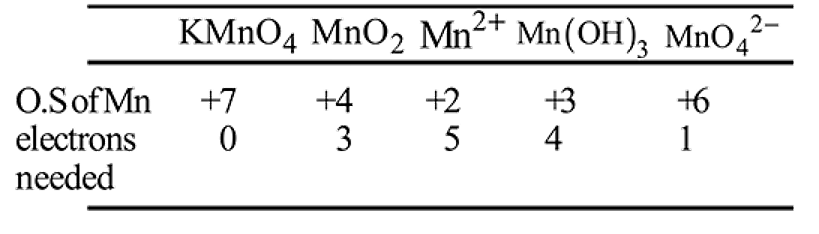
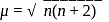

 we find
we find  No. of unpaired electrons
No. of unpaired electrons  hence its configuration will be
hence its configuration will be 

 Its chloride has the formula
Its chloride has the formula 
 is
is  . Being transition metal it has 7 valence electrons and all are involved in bond formation in
. Being transition metal it has 7 valence electrons and all are involved in bond formation in  . Hence it has no unpaired electron.
. Hence it has no unpaired electron.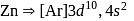
 means removal of electron from the stable configuration of
means removal of electron from the stable configuration of 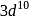 .
. . Hence, the correct option is (b).
. Hence, the correct option is (b). and
and 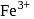 involve isoelectronic ions.
involve isoelectronic ions.

 Equivalent weight
Equivalent weight  Atomic weight
Atomic weight














