JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Oxidation and Reduction Reactions, Oxidation number - JEE MCQ
Test: Oxidation and Reduction Reactions, Oxidation number - JEE MCQ
Test Description
10 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Oxidation and Reduction Reactions, Oxidation number
Test: Oxidation and Reduction Reactions, Oxidation number for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Oxidation and Reduction Reactions, Oxidation number questions and answers have been
prepared according to the JEE exam syllabus.The Test: Oxidation and Reduction Reactions, Oxidation number MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Oxidation and Reduction Reactions, Oxidation number below.
Solutions of Test: Oxidation and Reduction Reactions, Oxidation number questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Oxidation and Reduction Reactions, Oxidation number solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Oxidation and Reduction Reactions, Oxidation number | 10 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Test: Oxidation and Reduction Reactions, Oxidation number - Question 1
Which one of the following cannot function as an oxidising agent?
Detailed Solution for Test: Oxidation and Reduction Reactions, Oxidation number - Question 1
Test: Oxidation and Reduction Reactions, Oxidation number - Question 2
Which of the following chemical reactions depict the oxidizing beahviour of  ?
?
Detailed Solution for Test: Oxidation and Reduction Reactions, Oxidation number - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Oxidation and Reduction Reactions, Oxidation number - Question 3
Which reaction involves neither oxidation nor reduction?
Detailed Solution for Test: Oxidation and Reduction Reactions, Oxidation number - Question 3
Test: Oxidation and Reduction Reactions, Oxidation number - Question 4
One gas bleaches the colour of flowers by reduction, while the other by oxidation
Detailed Solution for Test: Oxidation and Reduction Reactions, Oxidation number - Question 4
Test: Oxidation and Reduction Reactions, Oxidation number - Question 5
In how many of the following species, an element has fractional oxidation state?


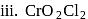
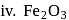




Detailed Solution for Test: Oxidation and Reduction Reactions, Oxidation number - Question 5
Test: Oxidation and Reduction Reactions, Oxidation number - Question 6
The most powerful reducing agent is:
Detailed Solution for Test: Oxidation and Reduction Reactions, Oxidation number - Question 6
Test: Oxidation and Reduction Reactions, Oxidation number - Question 7
 On balancing the above equation in basic solution, using integral coefficient, which of the following whole numbers will be the coefficient of
On balancing the above equation in basic solution, using integral coefficient, which of the following whole numbers will be the coefficient of  ?
?
Detailed Solution for Test: Oxidation and Reduction Reactions, Oxidation number - Question 7
Test: Oxidation and Reduction Reactions, Oxidation number - Question 8
In  the oxidation states respectively for
the oxidation states respectively for  and oxygen may be:
and oxygen may be:
 the oxidation states respectively for
the oxidation states respectively for  and oxygen may be:
and oxygen may be:
Detailed Solution for Test: Oxidation and Reduction Reactions, Oxidation number - Question 8
Test: Oxidation and Reduction Reactions, Oxidation number - Question 9



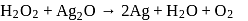
Role of hydrogen peroxide in the above reactions is respectively
Detailed Solution for Test: Oxidation and Reduction Reactions, Oxidation number - Question 9
Test: Oxidation and Reduction Reactions, Oxidation number - Question 10
Oxidation number of potassium in  and
and  , respectively:
, respectively:
 and
and  , respectively:
, respectively:
Detailed Solution for Test: Oxidation and Reduction Reactions, Oxidation number - Question 10
|
352 videos|596 docs|309 tests
|
Information about Test: Oxidation and Reduction Reactions, Oxidation number Page
In this test you can find the Exam questions for Test: Oxidation and Reduction Reactions, Oxidation number solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Oxidation and Reduction Reactions, Oxidation number, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF



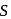 is decreasing from
is decreasing from  to
to 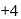 hence undergoing reduction and for HI oxidation Number of I is increasing from
hence undergoing reduction and for HI oxidation Number of I is increasing from  to 0 hence underegoing oxidation therefore
to 0 hence underegoing oxidation therefore 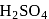 is acting as oxidising agent.
is acting as oxidising agent. .
.  . of
. of  on both sides is
on both sides is  .
. bleaches by reduction, while chlorine bleaches colour of flowers by oxidation.
bleaches by reduction, while chlorine bleaches colour of flowers by oxidation. oxidation state of P is+3 , which can further be oxidized to a higher oxidation state. So they can act as reducing agents. In
oxidation state of P is+3 , which can further be oxidized to a higher oxidation state. So they can act as reducing agents. In  oxidation state of P is +5, which is highest for P. Hence
oxidation state of P is +5, which is highest for P. Hence  cannot serve as a reducing agent.
cannot serve as a reducing agent.


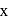 be the oxidation state of
be the oxidation state of  in
in 
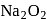 contains a peroxide linkage in which
contains a peroxide linkage in which  has an
has an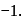
 or
or  or
or 
 is
is 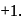
 and
and  are reduced.
are reduced. 

 (oxide) is -2, and in
(oxide) is -2, and in  (peroxide) is -1 and in
(peroxide) is -1 and in  (superoxide) is
(superoxide) is 















