Test: Purification of Organic Compounds - JEE MCQ
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Purification of Organic Compounds
For the separation of two miscible liquids which method is used?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In Lassaigne's test, the organic compound is fused with a piece of sodium metal in order to
A is a lighter phenol and B is an aromatic carboxylic acid. Separation of mixture of A and B can be carried out easily by using a solution of
In Dumas method, 0.3 g of an organic compound gave 45 mL of nitrogen at STP. The percentage of nitrogen is
The Lassaigne's extract is boiled with dil. HNO3 before testing for halogens because
Beilstein test is used for determination of
2.79 g of an organic compound when heated in Carius tube with conc. HNO3 and H3PO4 formed is converted into MgNH4.PO4 ppt. The ppt. on heating gave 1.332 g of Mg2P2O7. The percentage of P in the compound is
If 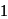 equivalent
equivalent 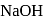 reacts with the given compound:
reacts with the given compound:
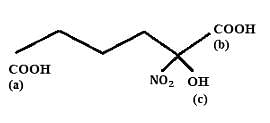
Choose the correct option:
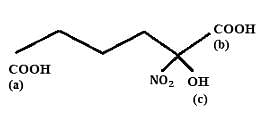
Find out the double bond equivalent (DBE) value of the given following compound:
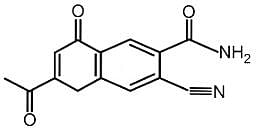
The prussian blue colour obtained during the test of nitrogen by Lassaigne's test is due to the formation of :
The purification of naphthalene can be done by
Two substances having slightly different solubility can be separated by
Which of the following statements is true regarding the fractional distillation of a mixture of two liquids?
A compound which decomposes before its boiling point can be purified by the method of
Chromatography is based on the phenomenon of
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|


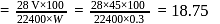
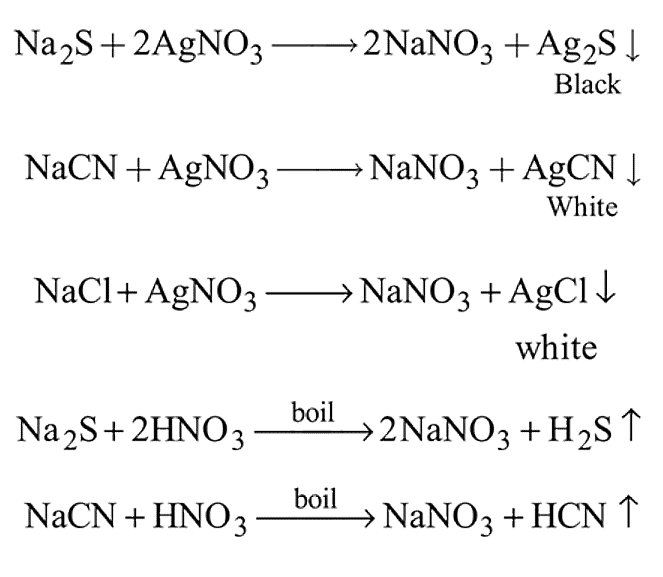
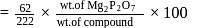
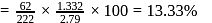
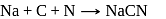
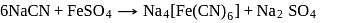
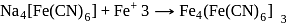
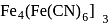 is responsible for prussian blue colour obtained during the test of Nitrogen and Lassaigne test. O-nitrophenal is more volatile than pnitrophenal. There is intramolecular H - bonding in O- nitrophenal while in P- nitrophenal there is intermolecular hydrogen bonding which cause association of molecule. Intermolecular H - bondingincrease, boiling point increases while volatility reduces.
is responsible for prussian blue colour obtained during the test of Nitrogen and Lassaigne test. O-nitrophenal is more volatile than pnitrophenal. There is intramolecular H - bonding in O- nitrophenal while in P- nitrophenal there is intermolecular hydrogen bonding which cause association of molecule. Intermolecular H - bondingincrease, boiling point increases while volatility reduces.














