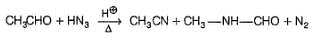Test: Reactions With Amines, Ammonia, Hydrazine & Its Derivatives - NEET MCQ
21 Questions MCQ Test Topic-wise MCQ Tests for NEET - Test: Reactions With Amines, Ammonia, Hydrazine & Its Derivatives
Only One Option Correct Type
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Arrange the following in the increasing order of reactivity with NH3.
I. CH2O
II. CH3CHO
III. CH3—CO—CH3
II. CH3CHO
III. CH3—CO—CH3
The major organic product formed in the following reaction is
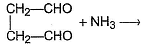
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which carbonyl compound below gives the reddish orange precipitate with 2, 4-dinitrophenyl hydrazine?
Which carbonyl compound below forms a single semicarbazone when allowed to react with semicarbazide?
A hydrocarbon X (C7H14) on ozonolysis followed by work-up with Zn- H2O gives C6H12O as one of the product which on subsequent treatment with KOH/I2 gives yellow precipitate and salt of a chiral acid. Hence, X could be
Which gives more than one oximes on treatment with hydroxylamine?
Which forms an unsaturated amine on treatment with cyclopentanone in slightly acidic medium?
Consider the following reaction,
Q.
X (major) in the above reaction is
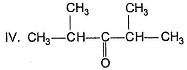
What is the final major product Y in the following reaction?
What is the final major product in the following reaction?
One or More than One Options Correct Type
Direction (Q. Nos. 11-14) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
What is/are expected product(s) in the following reaction?
Which of the following form enamine on heating with a secondary amine in weakly acidic medium ?
The compound(s) which form a pair of diastereomers with hydroxylamine is/are
The compound(s) below that gives yellow precipitate with KOH/I2 is/are
Comprehension Type
Direction (Q. Nos. 15-17) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Aidehydes and ketones react with ammonia and primary amines to form imines in slightly acidic condition. A typical reaction mechanism with a primary amine is as follows
The proper pH control is crucial to imine formation otherwise reaction does not proceed at appreciable rate.
However, if a secondary amine is reacted, no hydrogen is left on nitrogen on intermediate (I) for the deprotonation in step II. Hence, the reaction proceeds as
Enamine is a suitable intermediate route for α-alkyiation of a carbonyl compound.
Q.
What happens if imine form ation is carried out at very low pH?
Aidehydes and ketones react with ammonia and primary amines to form imines in slightly acidic condition. A typical reaction mechanism with a primary amine is as follows
The proper pH control is crucial to imine formation otherwise reaction does not proceed at appreciable rate.
However, if a secondary amine is reacted, no hydrogen is left on nitrogen on intermediate (I) for the deprotonation in step II. Hence, the reaction proceeds as
Enamine is a suitable intermediate route for α-alkyiation of a carbonyl compound.
Q.
Which of the following is expected to give more than one imine when treated with CH3NH2?
Aidehydes and ketones react with ammonia and primary amines to form imines in slightly acidic condition. A typical reaction mechanism with a primary amine is as follows
The proper pH control is crucial to imine formation otherwise reaction does not proceed at appreciable rate.
However, if a secondary amine is reacted, no hydrogen is left on nitrogen on intermediate (I) for the deprotonation in step II. Hence, the reaction proceeds as
Enamine is a suitable intermediate route for α-alkyiation of a carbonyl compound.
Q.
What is the product X in the following reaction?
One Integer Value Correct Type
Direction (Q. Nos. 18-21) This section contains 4 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
In the following reaction, how many isomers of trioximes are formed?
Consider the isomeric aldehydes with molar mass 100, if all the isomers (only structural) are treated independently with NH2OH, how many of them would give more than two stereomeric oximes?
When formaldehyde reacts with ammonia, a typical compound called hexamethylene tetramine is formed. How many six membered rings are present in this compound?
The smallest acyclic ketone that gives pair of diastereomers with CH3NH2 in slightly acidic medium has how many carbon atoms?
|
9 docs|1272 tests
|