JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Separation of mixture of amines - JEE MCQ
Test: Separation of mixture of amines - JEE MCQ
Test Description
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Separation of mixture of amines
Test: Separation of mixture of amines for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Separation of mixture of amines questions and answers have been
prepared according to the JEE exam syllabus.The Test: Separation of mixture of amines MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Separation of mixture of amines below.
Solutions of Test: Separation of mixture of amines questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Separation of mixture of amines solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Separation of mixture of amines | 15 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Test: Separation of mixture of amines - Question 1
The product of an amine '  ' with benzene sulphonyl chloride produces the product which is insoluble in alkali. The product of '
' with benzene sulphonyl chloride produces the product which is insoluble in alkali. The product of '  ' with ethanoyl chloride is
' with ethanoyl chloride is
 ' with benzene sulphonyl chloride produces the product which is insoluble in alkali. The product of '
' with benzene sulphonyl chloride produces the product which is insoluble in alkali. The product of '  ' with ethanoyl chloride is
' with ethanoyl chloride is
Detailed Solution for Test: Separation of mixture of amines - Question 1
Test: Separation of mixture of amines - Question 2
When diazonium salt solution is treated with water at a temperature of 283 K it forms?
Detailed Solution for Test: Separation of mixture of amines - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Separation of mixture of amines - Question 3
Total number of reagents among the following which can reduce  in to ethyl amine.
in to ethyl amine.
 ,
,

Detailed Solution for Test: Separation of mixture of amines - Question 3
Test: Separation of mixture of amines - Question 4
Total number of compounds which are more basic than aniline.


Detailed Solution for Test: Separation of mixture of amines - Question 4
Test: Separation of mixture of amines - Question 5
Which one of the following compounds forms a quaternary salt on reacting with excess methyl iodide?
Detailed Solution for Test: Separation of mixture of amines - Question 5
Test: Separation of mixture of amines - Question 6
 has how many isomeric forms that contain a benzene ring?
has how many isomeric forms that contain a benzene ring?
Detailed Solution for Test: Separation of mixture of amines - Question 6
Test: Separation of mixture of amines - Question 7
The amine that does not react with acetyl chloride is
Detailed Solution for Test: Separation of mixture of amines - Question 7
Test: Separation of mixture of amines - Question 8
In the diazotisation of arylamines with sodium nitrite and hydrochloric acid, an excess of hydrochloric acid is used primarily to
Detailed Solution for Test: Separation of mixture of amines - Question 8
Test: Separation of mixture of amines - Question 9
Nitrosoamines  are insoluble in water. On heating with conc.
are insoluble in water. On heating with conc.  , they give secondary amines. The reaction is called
, they give secondary amines. The reaction is called
 are insoluble in water. On heating with conc.
are insoluble in water. On heating with conc.  , they give secondary amines. The reaction is called
, they give secondary amines. The reaction is called
Detailed Solution for Test: Separation of mixture of amines - Question 9
Test: Separation of mixture of amines - Question 10
Which of the following reactions can produce aniline as main product?
Detailed Solution for Test: Separation of mixture of amines - Question 10
Test: Separation of mixture of amines - Question 11
In a reaction of aniline a coloured product  was obtained.
was obtained.

The structure of C would be:
Detailed Solution for Test: Separation of mixture of amines - Question 11
Test: Separation of mixture of amines - Question 12
Statement I: Aldehyde on reaction with  gives Cyanohydrin.
gives Cyanohydrin.
Statement II: Cyanohydrin is a compound which consists of hydroxy and cyano groups on the same carbon.
Choose the correct answer from the following with reference to above statements.
 gives Cyanohydrin.
gives Cyanohydrin.Statement II: Cyanohydrin is a compound which consists of hydroxy and cyano groups on the same carbon.
Choose the correct answer from the following with reference to above statements.
Detailed Solution for Test: Separation of mixture of amines - Question 12
Test: Separation of mixture of amines - Question 13
 -Chloroaniline and anilinium hydrogen chloride can be distinguished by:
-Chloroaniline and anilinium hydrogen chloride can be distinguished by:
Detailed Solution for Test: Separation of mixture of amines - Question 13
Test: Separation of mixture of amines - Question 14
Primary amines can be distinguished from secondary and tertiary amines by reacting with
Detailed Solution for Test: Separation of mixture of amines - Question 14
Test: Separation of mixture of amines - Question 15
A mixture containing the following four compounds is extracted with 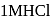 . The compound that goes to aqueous layer is :
. The compound that goes to aqueous layer is :

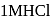 . The compound that goes to aqueous layer is :
. The compound that goes to aqueous layer is : 
Detailed Solution for Test: Separation of mixture of amines - Question 15
|
352 videos|596 docs|309 tests
|
Information about Test: Separation of mixture of amines Page
In this test you can find the Exam questions for Test: Separation of mixture of amines solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Separation of mixture of amines, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF



 ' will be
' will be
 and
and 

 having one benzene ring may be represented as
having one benzene ring may be represented as  , may be in the form of
, may be in the form of  and
and  amines in the following five isomeric forms.
amines in the following five isomeric forms.
 -atoms
-atoms  atoms attached to
atoms attached to  or
or  react with
react with  to form acetyl derivatives.
to form acetyl derivatives.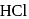 is used to convert free aniline to aniline hydrochloride otherwise free aniline would undergo coupling reaction with benzenediazonium chloride.
is used to convert free aniline to aniline hydrochloride otherwise free aniline would undergo coupling reaction with benzenediazonium chloride.



 -Chloroaniline and anilinium hydrogen chloride can be distinguished by
-Chloroaniline and anilinium hydrogen chloride can be distinguished by  . Anilinium hydrogen chloride will give white ppt of
. Anilinium hydrogen chloride will give white ppt of  on reaction with
on reaction with 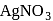 , but
, but 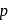 -chloronoaniline will not react with it because
-chloronoaniline will not react with it because 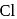 is directly attached to benzene nucleus.
is directly attached to benzene nucleus. and alc.
and alc.  to form isocyanide while secondary and tertiary amines do not react
to form isocyanide while secondary and tertiary amines do not react
 , amine get protonated and becomes
, amine get protonated and becomes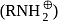 , which does not dissolve in organic solvent but usually dissolve in
, which does not dissolve in organic solvent but usually dissolve in  due to its charge. So, shaking with aqueous HCl will pull amines into the aqueous phase and leave all other compounds in organic layer.
due to its charge. So, shaking with aqueous HCl will pull amines into the aqueous phase and leave all other compounds in organic layer.














