Test: general properties of hydrocarbons - JEE MCQ
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: general properties of hydrocarbons
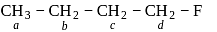
Arrange the hydrogens a, b, c, d in decreasing order of their reactivities towards chlorination:
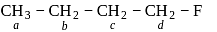
Arrange the hydrogens a, b, c, d in decreasing order of their reactivities towards chlorination:
Among the following free radical bromination reactions, select those in which 2o halide is the major product -


| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following is not expected to be intermediate of the following reaction?


The compound prepared by a substitution reaction of benzene is
The order of increasing reactivity of the following compounds towards HCl will be

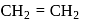




Consider the following reaction
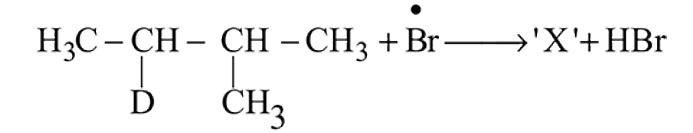
Identify the structure of the major product '  '
'
Free radical chlorination of hexane produces how many of monochloro derivatives? (including stereoisomer)
In preparation of alkene from alcohol using Al2O3 which is effective factor?
When 1, 2 - dibromoethane reacts with sodamide giving a product X. The hybridisation state of the carbons present in it respectively, are
How many monochlorobutanes will be obtained on chlorination of n -butane? Consider only structural isomers.
A hydrocarbon with formula C8H18 gives one monochloro derivative. The hydrocarbon is
The major product obtained in the dehydrohalogenaton of neopentylbromide with alcoholic KOH is
The major products obtained in the following reaction is/are

A hydrocarbon contains 10.5 g carbon and 1 g hydrogen. Its 2.8 g has 1 L volume at 1 atm and 127oC, what is the number of carbon in the molecular formula of given hydrocarbon?
Which of the following reaction produce alkane as the product?
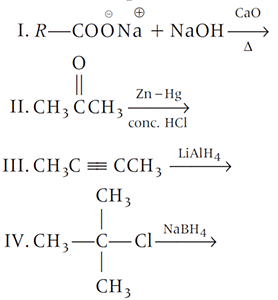
Which one of the following reactions is expected to readily give a hydrocarbon product in good yields?

Double bond equivalent (degree of unsaturation) of (A) is:
The cycloalkane having the lowest heat of combustion per CH2 group
Which of the following cannot be considered as a step of mechanism in the chain reaction of methane with Cl2?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|






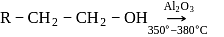



 one monochlorintaion product.
one monochlorintaion product.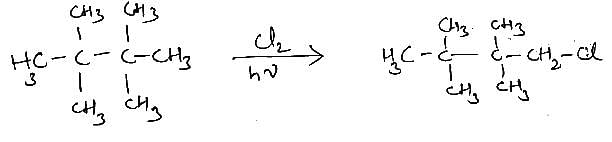

 Cis T
Cis T  Trans
Trans Anti A
Anti A  Anti
Anti Racemic M
Racemic M  Meso
Meso








 is mainly –
is mainly –















