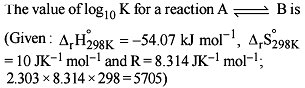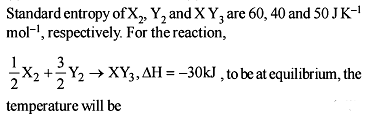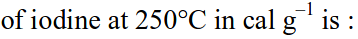Thermodynamics - 2 - JEE MCQ
30 Questions MCQ Test Chemistry for JEE Main & Advanced - Thermodynamics - 2
3 moles of a diatomic gas are heated from 127° C to 727° C at a constant pressure of 1 atm. Entropy change is (log 2.5 = 0 .4)
Exactly 100 J of heat was transferred reversibly to a block of gold at 25.00° C from a thermal reservoir at 25.01 °C, and then exactly 100 J of heat was absorbed reversibled from the block of gold by a thermal reservoir at 24.99° C. Thus entropy change of the system is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Consider a reversible isentropic expansion of 1.0 mole of an ideal monoatomic gas from 25°C to 75°C. If the initial pressure was 1.0 bar, final pressure is
For the process, and 1 atmosphere pressure, the correct choice is
[JEE Advanced 2014]
Benzene and naphthalene form an ideal solution at room temperature. For this process, the true statement(s) is (are)
[JEE Advanced 2013]
Which one of the following equations does not correctly represent the first law of thermodynamics for the given processes involving an ideal gas ? (Assume non-expansion work is zero)
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|