JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Test: Nomenclature of coordination compounds - JEE MCQ
Test: Nomenclature of coordination compounds - JEE MCQ
Test Description
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Nomenclature of coordination compounds
Test: Nomenclature of coordination compounds for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Test: Nomenclature of coordination compounds questions and answers have been
prepared according to the JEE exam syllabus.The Test: Nomenclature of coordination compounds MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Nomenclature of coordination compounds below.
Solutions of Test: Nomenclature of coordination compounds questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Test: Nomenclature of coordination compounds solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Test: Nomenclature of coordination compounds | 20 questions in 20 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Detailed Solution for Test: Nomenclature of coordination compounds - Question 1
Test: Nomenclature of coordination compounds - Question 2
select the correct IUPAC name for the following:
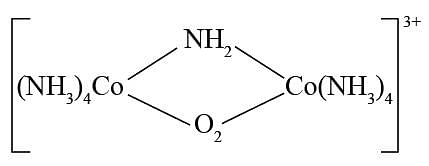
Detailed Solution for Test: Nomenclature of coordination compounds - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Detailed Solution for Test: Nomenclature of coordination compounds - Question 3
Test: Nomenclature of coordination compounds - Question 4
The correct IUPAC name of the complex:
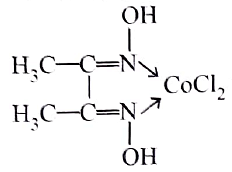
is

is
Detailed Solution for Test: Nomenclature of coordination compounds - Question 4
Test: Nomenclature of coordination compounds - Question 5
Coordination number and oxidation state of  in
in 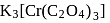 are, respectively:
are, respectively:
 in
in 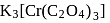 are, respectively:
are, respectively:
Detailed Solution for Test: Nomenclature of coordination compounds - Question 5
Detailed Solution for Test: Nomenclature of coordination compounds - Question 6
Test: Nomenclature of coordination compounds - Question 7
Which of the following is not a complex salt 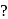
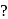
Detailed Solution for Test: Nomenclature of coordination compounds - Question 7
Detailed Solution for Test: Nomenclature of coordination compounds - Question 8
Test: Nomenclature of coordination compounds - Question 9
Red precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammoniacal Ni(II). Which of the following statements is not true?
Detailed Solution for Test: Nomenclature of coordination compounds - Question 9
Test: Nomenclature of coordination compounds - Question 10
The octahedral complex of a metal ion 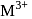 with four monodentate ligands
with four monodentate ligands 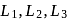 and
and  absorb wavelengths in the region of red, green, yellow and blue, respectively. The increasing order of ligand strength of the four ligands is:
absorb wavelengths in the region of red, green, yellow and blue, respectively. The increasing order of ligand strength of the four ligands is:
Detailed Solution for Test: Nomenclature of coordination compounds - Question 10
Test: Nomenclature of coordination compounds - Question 11
Which one of the following coordination compounds is used to inhibit the growth of tumours?
Detailed Solution for Test: Nomenclature of coordination compounds - Question 11
Test: Nomenclature of coordination compounds - Question 12
The EAN of iron in potassium ferricyanide is
Detailed Solution for Test: Nomenclature of coordination compounds - Question 12
Test: Nomenclature of coordination compounds - Question 13
AgCl is soluble in 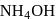 solution. The solubility is due to formation of
solution. The solubility is due to formation of
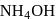 solution. The solubility is due to formation of
solution. The solubility is due to formation of
Detailed Solution for Test: Nomenclature of coordination compounds - Question 13
Test: Nomenclature of coordination compounds - Question 14
In which of the following coordinate compounds the central metal atom obeys the EAN rule.
Detailed Solution for Test: Nomenclature of coordination compounds - Question 14
Test: Nomenclature of coordination compounds - Question 15
Which of the following will exhibit maximum ionic conductivity?
Detailed Solution for Test: Nomenclature of coordination compounds - Question 15
Test: Nomenclature of coordination compounds - Question 16
Which of the following complexes have a maximum number of unpaired electrons?
Detailed Solution for Test: Nomenclature of coordination compounds - Question 16
Test: Nomenclature of coordination compounds - Question 17
The degeneracy of 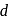 -orbitals is lost under
-orbitals is lost under
(I) Strong field ligand
(II) Weak field ligand
(III) Mixed field ligand
(IV) Chelated ligand field
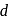 -orbitals is lost under
-orbitals is lost under(I) Strong field ligand
(II) Weak field ligand
(III) Mixed field ligand
(IV) Chelated ligand field
Detailed Solution for Test: Nomenclature of coordination compounds - Question 17
Test: Nomenclature of coordination compounds - Question 18
The IUPAC name of the following complex is 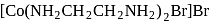
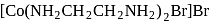
Detailed Solution for Test: Nomenclature of coordination compounds - Question 18
Test: Nomenclature of coordination compounds - Question 19
Which of the following name formula combinations is not correct?

Detailed Solution for Test: Nomenclature of coordination compounds - Question 19
Test: Nomenclature of coordination compounds - Question 20
In  , the number of covalent bonds is
, the number of covalent bonds is
 , the number of covalent bonds is
, the number of covalent bonds is
Detailed Solution for Test: Nomenclature of coordination compounds - Question 20
|
352 videos|596 docs|309 tests
|
Information about Test: Nomenclature of coordination compounds Page
In this test you can find the Exam questions for Test: Nomenclature of coordination compounds solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Nomenclature of coordination compounds, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF


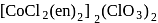 .
. -Amido-
-Amido- 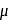 -peroxidobis(tetraammine)dicobalt-(III) ion
-peroxidobis(tetraammine)dicobalt-(III) ion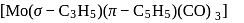
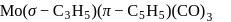 :
: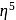 - cyclopentadienyl) molybdenum (II).
- cyclopentadienyl) molybdenum (II).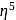 represents that the cyclopnetadienyl ligand shares 5 electrons with metal.
represents that the cyclopnetadienyl ligand shares 5 electrons with metal.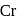 is 6 .
is 6 .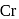 be
be 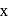 .
.

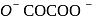 ?
?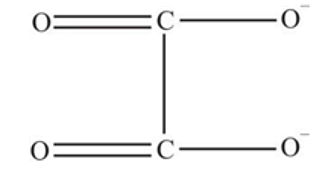
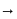 oxalate ion is bidentate due to two donor atom
oxalate ion is bidentate due to two donor atom 
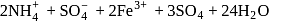
 - bonded complex ?
- bonded complex ? is not
is not 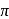 bonded complex. It is
bonded complex. It is 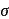 bonded organometallic compound
bonded organometallic compound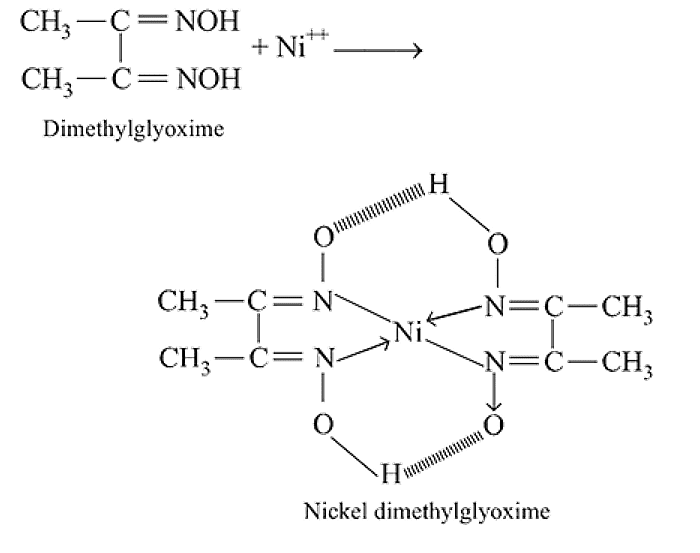
 , which will absorbs light of longer
, which will absorbs light of longer 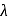 and thus lower frequency. Conservely, stronger field ligands create a larger
and thus lower frequency. Conservely, stronger field ligands create a larger 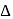 , absorb light of shorter
, absorb light of shorter 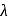 and thus higher
and thus higher 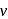 i.e. higher energy.
i.e. higher energy.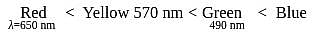

 which is
which is 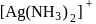
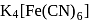 obeys the EAN rule.
obeys the EAN rule.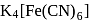 in solution furnishes
in solution furnishes 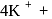
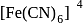 five ions and exhibits maximum ionic conductivity.
five ions and exhibits maximum ionic conductivity.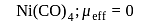
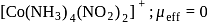
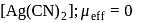

 -orbitals undergo splitting under ligand field created by strong, weak or mixed ligands.
-orbitals undergo splitting under ligand field created by strong, weak or mixed ligands.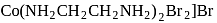 is dibromidobis (ethylene diamine) cobalt (III) bromide.
is dibromidobis (ethylene diamine) cobalt (III) bromide.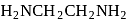 is ethylene diamine which is a bidentate ligand.
is ethylene diamine which is a bidentate ligand.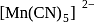 Pentacyanomangnate (III).
Pentacyanomangnate (III). and
and  . Therefore the number of covalent bonds is 18 .
. Therefore the number of covalent bonds is 18 .














