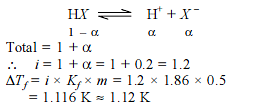JEE Exam > JEE Tests > Chemistry for JEE Main & Advanced > Liquid state - 2 - JEE MCQ
Liquid state - 2 - JEE MCQ
Test Description
30 Questions MCQ Test Chemistry for JEE Main & Advanced - Liquid state - 2
Liquid state - 2 for JEE 2024 is part of Chemistry for JEE Main & Advanced preparation. The Liquid state - 2 questions and answers have been
prepared according to the JEE exam syllabus.The Liquid state - 2 MCQs are made for JEE 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Liquid state - 2 below.
Solutions of Liquid state - 2 questions in English are available as part of our Chemistry for JEE Main & Advanced for JEE & Liquid state - 2 solutions in
Hindi for Chemistry for JEE Main & Advanced course. Download more important topics, notes, lectures and mock
test series for JEE Exam by signing up for free. Attempt Liquid state - 2 | 30 questions in 60 minutes | Mock test for JEE preparation | Free important questions MCQ to study Chemistry for JEE Main & Advanced for JEE Exam | Download free PDF with solutions
Detailed Solution for Liquid state - 2 - Question 1
Detailed Solution for Liquid state - 2 - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Liquid state - 2 - Question 3
Molal depression constant for a solvent is 4.0 K kg mol–1. The depression in the freezing point of the solvent for 0.03 mol kg–1 solution of K2OS4 is : (Assume complete dissociation of the electrolyte)
Detailed Solution for Liquid state - 2 - Question 3
Detailed Solution for Liquid state - 2 - Question 4
Detailed Solution for Liquid state - 2 - Question 5
Detailed Solution for Liquid state - 2 - Question 6
Detailed Solution for Liquid state - 2 - Question 7
Detailed Solution for Liquid state - 2 - Question 8
Detailed Solution for Liquid state - 2 - Question 9
Liquid state - 2 - Question 10
The vapour pressures of pure liquids A and B are 400 and 600 mmHg, respectively at 298 K. On mixing the two liquids, the sum of their initial volumes is equal to the volume of the final mixture. The mole fraction of liquid B is 0.5 in the mixture. The vapour pressure of the final solution, the mole fractions of components A and B in vapour phase, respectively are -
Detailed Solution for Liquid state - 2 - Question 10
Detailed Solution for Liquid state - 2 - Question 11
Detailed Solution for Liquid state - 2 - Question 12
Detailed Solution for Liquid state - 2 - Question 13
Detailed Solution for Liquid state - 2 - Question 14
Detailed Solution for Liquid state - 2 - Question 15
Detailed Solution for Liquid state - 2 - Question 16
Detailed Solution for Liquid state - 2 - Question 17
Detailed Solution for Liquid state - 2 - Question 18
Detailed Solution for Liquid state - 2 - Question 19
Detailed Solution for Liquid state - 2 - Question 20
Detailed Solution for Liquid state - 2 - Question 21
Detailed Solution for Liquid state - 2 - Question 22
Detailed Solution for Liquid state - 2 - Question 23
Detailed Solution for Liquid state - 2 - Question 24
Detailed Solution for Liquid state - 2 - Question 25
Liquid state - 2 - Question 26
Elevation of boiling point of 1 molar aqueous glucose solution (density = 1.2 g/ml) is
Detailed Solution for Liquid state - 2 - Question 26
Detailed Solution for Liquid state - 2 - Question 27
Detailed Solution for Liquid state - 2 - Question 28
Detailed Solution for Liquid state - 2 - Question 29
Detailed Solution for Liquid state - 2 - Question 30
|
352 videos|596 docs|309 tests
|
Information about Liquid state - 2 Page
In this test you can find the Exam questions for Liquid state - 2 solved & explained in the simplest way possible.
Besides giving Questions and answers for Liquid state - 2, EduRev gives you an ample number of Online tests for practice
|
352 videos|596 docs|309 tests
|
Download as PDF